42 zinc electron dot diagram
Lewis Electron Dot Diagram For Fluoride Ion The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond). The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. ELECTRON DOT DIAGRAMS - msduncanchem.com electron dot diagrams . element. electron configuration. noble gas configuration. highest occupied energy level
PDF Chemistry Lab: Electron Dot Diagrams for Atoms & Ions 2) For the electron dot diagrams, use one color per element and its dots. ELEMENTS TO USE IN THE DATA TABLE: 1) boron 2) silicon 3) sulfur 4) calcium 5) iodine 6) rubidium 7) argon 8) carbon 9) arsenic 10) chlorine 11) zinc 12) iridium #77 - typical transition metal, no exception

Zinc electron dot diagram
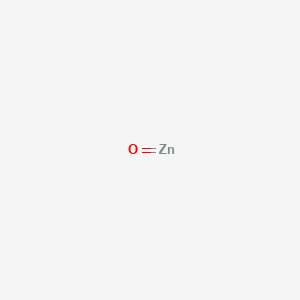
Zinc Chloride (ZnCl2) - Structure, Properties, & Uses of ... Zinc chloride is solid at room temperature and has a white crystalline appearance. It is odourless. The solubility of this compound in water corresponds to 432g/100g. It is also soluble in acetone, ethanol, and glycerol. The four polymorphs of ZnCl 2 feature a tetrahedral coordinate geometry between the Zn 2+ ions and the Cl - Draw the electron dot structure of the gas molecule which ... Draw the electron dot structure of the gas molecule which is liberated zinc metal is treated with aqueous NaOH solution. Medium. Open in App. Solution. Verified by Toppr. The gas released on reaction is Hydrogen. Solve any question of Chemical Bonding and Molecular Structure with:- Pourbaix Diagram Of Zinc Pdf Merger - heavenlytxt Pourbaix Diagram Of Zinc Pdf Merger 9/22/2020 Elemental Pourbaix Diagram. The two orange lines are the hydrogen reduction line, and the line denoting water oxidation to O 2. These are clearly labeled in Figure 3. These lines show the stability region of H 2 O.
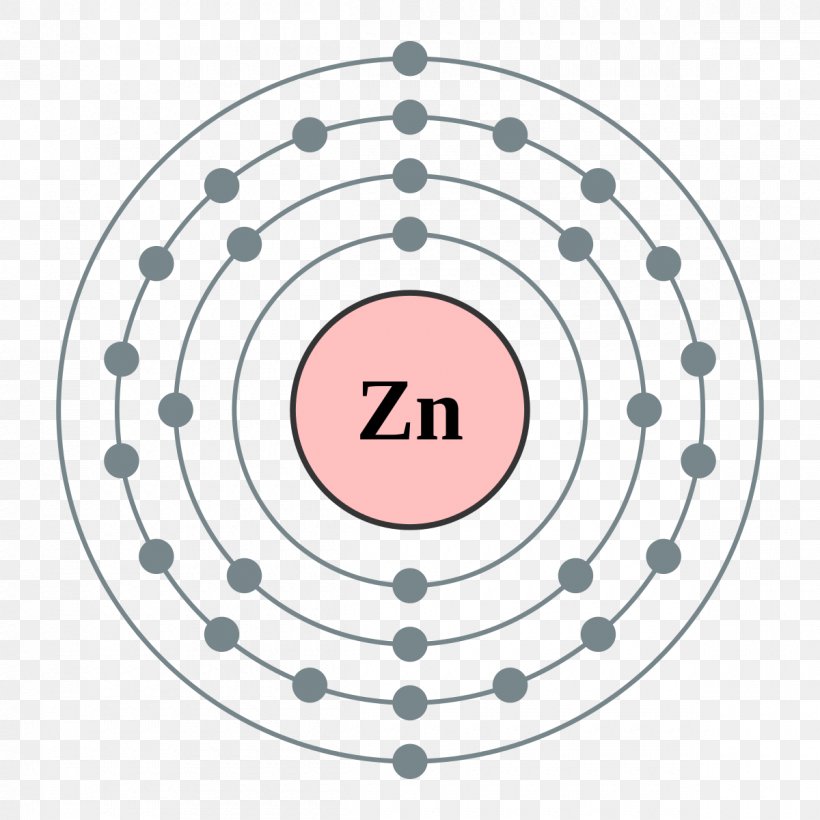
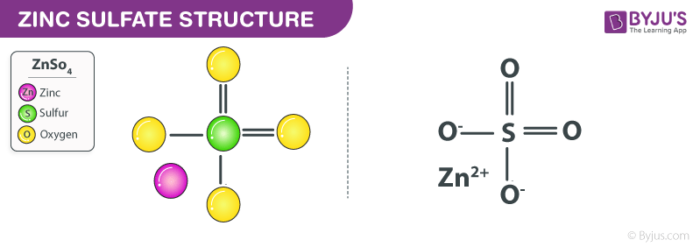
Zinc electron dot diagram. How does the Lewis dot structure of zinc look like? - Answers Zinc is #30. It has an electron configuration of [Ar] 4s2, 3d10. The 4s2 electrons are the bonding electrons. The dot structrue 2 dots. Zn: Electron Configuration - Practice Problems KEY - Google Docs h. zinc. 16. What electron-dot structure is shared by all noble gases except helium? 8 dots surround the symbol for the element. 17. List three elements that have the following electron-dot structure: Zinc Sulfate (ZnSO4) - Structure, Properties, Molecular ... Zinc Sulfate (ZnSO4) - Zinc Sulfate is the chemical name of ZnSO4. Visit BYJUS to study the uses, properties, structure of Zinc Sulfate (ZnSO4) explained by the chemistry experts. What is the electron dot diagram for zinc? - Answers You write Zn and then you put one dot on top (or bottom) and one on the left (or right) like this for example: Zn . . (the reason why you have 2 dots is because zinc has only two valence electrons)
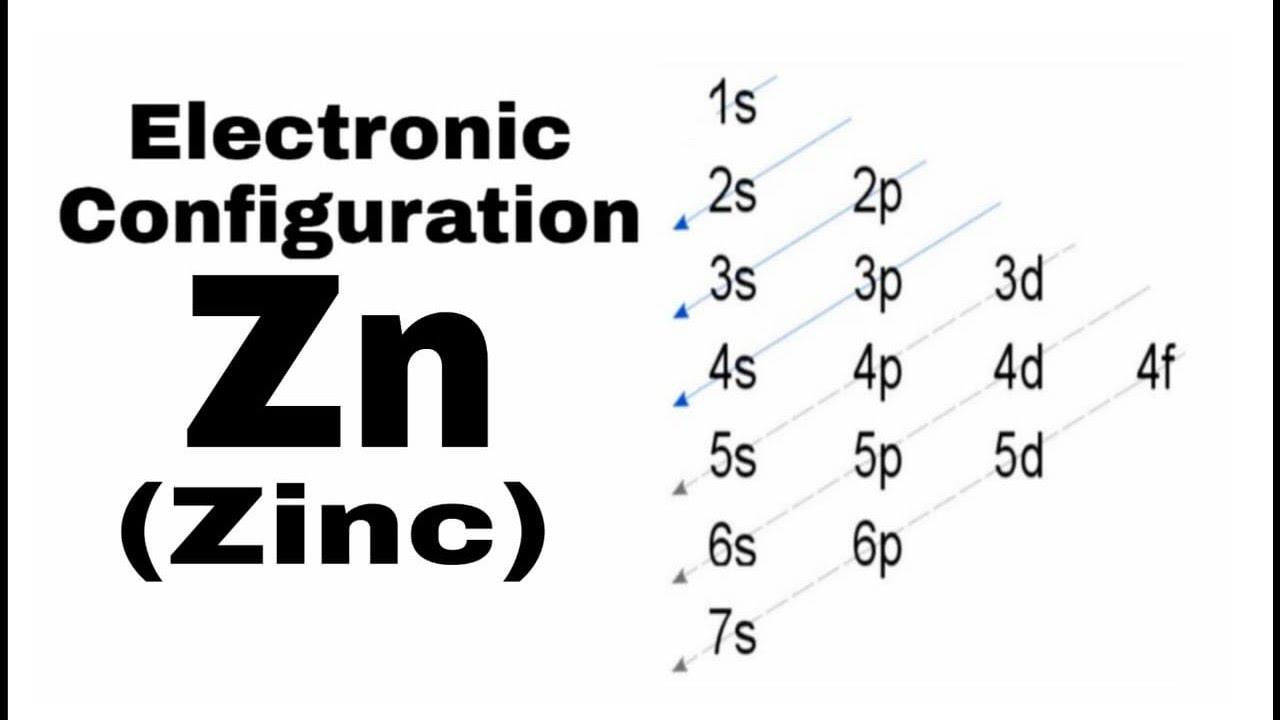
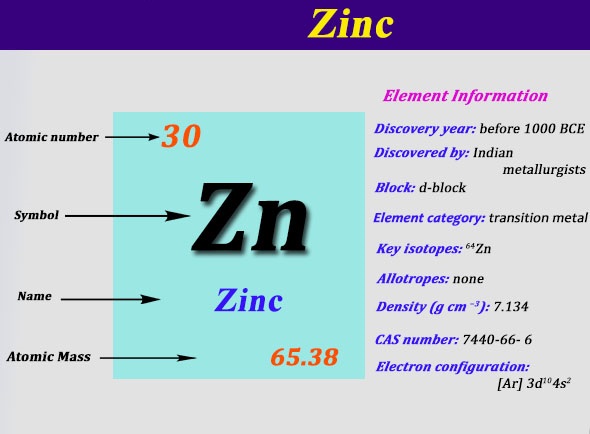
What is the electron dot diagram for zinc class 12 ... To find the electron dot diagram for zinc, we need to write its electronic configuration according to Aufbau's Principle. Aufbau's Principle: It states that the electrons must be filled first in orbitals with lower energy than the orbitals which have comparatively higher energy. Atomic number of Zinc = 30 PDF Electron Dot Diagrams for Ionic Compounds Lab - Kwanga.net 2) The steps to writing the electron dot diagram of a binary ionic compound: • Write the symbols of the elements (such as Na and Cl). • (Columns 1 & 4) Look up their oxidation numbers (charges) from their placement in the periodic table. Write the proper ion symbols and charges. • (Columns 2 and 5) Write the names of the ions. 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... How to draw ZnBr2 Lewis Structure? - Science Education and ... In the ZnBr2 Lewis structure diagram, we always begin by introducing valence electrons from the central Zinc atom. As a result, wrap around the central Zinc atom's bond pair valence electrons first. Zinc requires 4 electrons in its outermost valence shell to complete the molecular stability.
Zinc Oxide Formula - Molecular, Structure and Chemical Formula The chemical structure for the compound could be given as below. Zinc Oxide Chemical Formula. Its presence could be felt in nature and taken as the largest mineral in New Jersey USA. The compound has a hexagonal crystalline structure and there is just plenty of process to synthesize the zinc oxide. Draw the electron dot structure of the gas molecule which is ... Click here to get an answer to your question ✍️ Draw the electron dot structure of the gas molecule which is liberated zinc metal is treated with aqueous ...1 answer · Top answer: The gas released on reaction is Hydrogen. How to draw ZnCl2 Lewis Structure? - Science Education and ... In the ZnCl2 Lewis structure diagram, we always begin by introducing valence electrons from the central Zinc atom. As a result, wrap around the central Zinc atom's bond pair valence electrons first. Zinc requires 4 electrons in its outermost valence shell to complete the molecular stability. What is the electron dot diagram for zinc? | Socratic The electron dot diagram for zinc is "Zn:" > Zinc (element number 30) is in the 4th Period of the Periodic Table. From left to right, you count two "4s" electrons and ten "3d" electrons. The "3d" shell is a filled inner shell, so only the "4s" electrons are valence electrons. Thus, the electron dot structure for zinc is "Zn:"
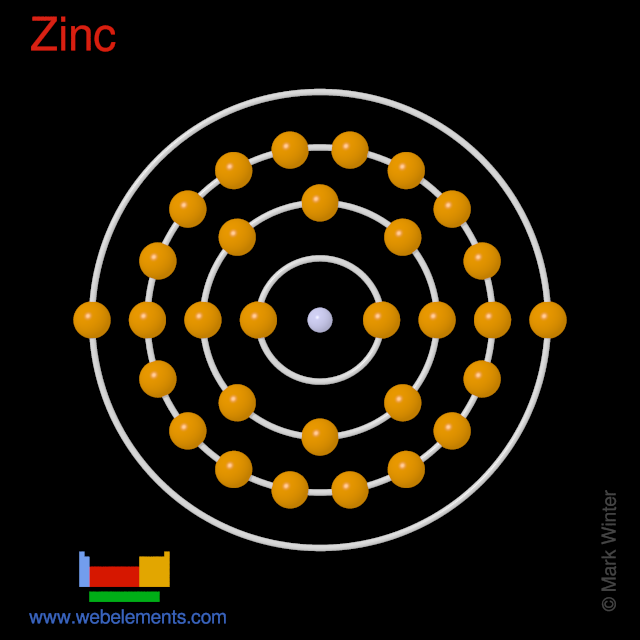
Diagrams - Zinc Diagrams - Zinc. Bohr Diagram- Valence Electrons: 2. . Electron Configuration. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10.
How to Draw the Lewis Dot Structure for Zn (Zinc) - YouTube A step-by-step explanation of how to draw the Zn Lewis Dot Structure.For the Zn structure use the periodic table to find the total number of valence electron...
ZINC permanganate | Mn2O8Zn - PubChem Zinc permanganate appears as a purplish colored crystalline solid. Noncombustible but accelerates burning of combustible material. Explosion hazard if the combustible material is finely divided. Contact with liquid combustible materials may result in spontaneous ignition. Contact with sulfuric acid may result in fires or explosions.
How to Draw the Lewis Dot Structure for ZnBr2 (Zinc ... A step-by-step explanation of how to draw the ZnBr2 Lewis Dot Structure.For ZnBr2 we have an ionic compound and we need to take that into account when we dra...
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
Lewis Dot Diagram - Organic Chemistry | Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
How many valence electrons does zinc(Zn) have? In this step, the electrons of zinc have to be arranged. We know that zinc atoms have a total of thirty electrons. The first two electrons enter the 1s orbital and the next two electrons enter the 2s orbital. The next six electrons enter the 2p orbital. The p-orbital can have a maximum of six electrons. So, six electrons enter the 2p orbital.
Lewis Dot Diagram For Gallium - schematron.org Draw a Lewis electron dot diagram for an atom or a monatomic ion. . b) gallium. 9. Draw the Lewis electron dot diagram for each ion. a) Mg 2+. b) S 2−. Comprehensive information for the element Gallium - Ga is provided by this page including scores of Atomic Structure of Gallium Electron Dot Model.
Periodic Table of Elements: Zinc - Zn ... Found in the minerals zinc blende (sphalerite) (ZnS), calamine, franklinite, smithsonite (ZnCO 3 ), willemite, and zincite (ZnO). Annual world wide production is around 5,020,000 tons. Primary mining areas are USA, Canada, Australia, Austria, Russia and Turkey. Uses of Zinc: Used to coat other metals (galvanizing) to protect them from rusting.
Zinc arsenide - Wikipedia Zinc arsenide (Zn 3 As 2) is a binary compound of zinc with arsenic which forms gray tetragonal crystals. It is an inorganic semiconductor with a band gap of 1.0 eV. Contents 1 Synthesis and reactions 2 Structure 3 Electronic structure 4 References Synthesis and reactions Zinc arsenide can be prepared by the reaction of zinc with arsenic
PDF Electron Dot (Lewis) Diagrams - Mr. Sault's Classroom It can also donate and electron at become positively charged. 4 Checklist 1. Draw an "electron dot" diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4.
ZINC chloride | ZnCl2 - PubChem Zinc chloride aerosol was lethal to rats at concentrations from 940 mg Zn2+/cu m (ca 1,975 mg ZnCl2/cu m), the animals died within 3 days after exposure. Microscopic findings in the lungs included atelectasis, hyperaemia, hemorrhages and edema, however without a clear-cut dose-response relationship.
Pourbaix Diagram Of Zinc Pdf Merger - heavenlytxt Pourbaix Diagram Of Zinc Pdf Merger 9/22/2020 Elemental Pourbaix Diagram. The two orange lines are the hydrogen reduction line, and the line denoting water oxidation to O 2. These are clearly labeled in Figure 3. These lines show the stability region of H 2 O.
Draw the electron dot structure of the gas molecule which ... Draw the electron dot structure of the gas molecule which is liberated zinc metal is treated with aqueous NaOH solution. Medium. Open in App. Solution. Verified by Toppr. The gas released on reaction is Hydrogen. Solve any question of Chemical Bonding and Molecular Structure with:-
Zinc Chloride (ZnCl2) - Structure, Properties, & Uses of ... Zinc chloride is solid at room temperature and has a white crystalline appearance. It is odourless. The solubility of this compound in water corresponds to 432g/100g. It is also soluble in acetone, ethanol, and glycerol. The four polymorphs of ZnCl 2 feature a tetrahedral coordinate geometry between the Zn 2+ ions and the Cl -








:max_bytes(150000):strip_icc()/Lead-58b601095f9b5860464ba934.jpg)







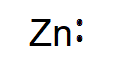









Comments
Post a Comment