42 orbital diagram for ti2+
Electron Configuration for Ti , Ti3+, and Ti4 ... - YouTube To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number... Electronic Structure of Atoms (Electron Configurations ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
What is the orbital diagram of TI? - AnswersToAll What is the correct orbital diagram for phosphorus? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s22s22p63s23p3.
Orbital diagram for ti2+
Orbital Diagram For Ti2 — UNTPIK APPS Orbital Diagram for Ti2. electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals What is the electron configuration of "Ti"^(2+)? | Socratic A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom.. In this case, titanium, #"Ti"#, is located in period 4, group 4 of the periodic table and has an atomic number of #22#. This means that a neutral titanium atom will contain #22# protons in its nucleus and #22# electrons surrounding its nucleus. Orbital Diagram of Titanium (Ti), electron configuration ... This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble...
Orbital diagram for ti2+. › 42397273 › Chemistry_the_centralChemistry the central science 14th edition - Academia.edu Academia.edu is a platform for academics to share research papers. OneClass: What is the orbital diagram of each atom or ion ... greenkangaroo798 Lv1. 28 Nov 2020. What is the orbital diagram of each atom or ion? Ti, Ti 2+, Ti 4+. Answer. + 20. Watch. 1. answer. (PDF) James E. Brady The Molecular Nature of Matter (6th ... James E. Brady The Molecular Nature of Matter (6th Edition) Copia Electron Configuration Calculator - Find element configuration It also describes every electron as moving freely in an orbital, in an average field generated by other orbitals. For example, electron configuration of Phosphorus (P) is 1s^2 2s^2 2p^6 3s^2 3p^3. Usually, Physicists and chemists use the standard notation to refer to the electronic configurations of molecules and atoms. For atoms, the standard ...
Exam 2 Flashcards - Quizlet Choose the ground state electron configuration for Ti2+ ... Choose the valence electron orbital diagram that represents the ground state of Se2-D (#1) The solid compound K2S2O3 contains. K+ ions and S2O3^2-Of the following, which atom has the largest atomic radius? K. Choose the best Lewis structure for SF4. E (#28) Titanium | Ti (Element) - PubChem Titanium is the ninth most abundant element in the earth's crust and is primarily found in the minerals rutile (TiO 2 ), ilmenite (FeTiO 3) and sphene (CaTiSiO 5 ). Titanium makes up about 0.57% of the earth's crust. Jefferson Lab, U.S. Department of Energy. From the Latin titans, the first sons of the Earth, Greek mythology. What is the electron configuration of Ti plus4? - Answers The electron configuration is the number of electrons in each energy level of an element. The electron configuration of Li is, 1s2 2s1. The electron configuration of F is, 1s2 2s2 2p5. Electron Configuration for Oxygen (O) - UMD Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be ...
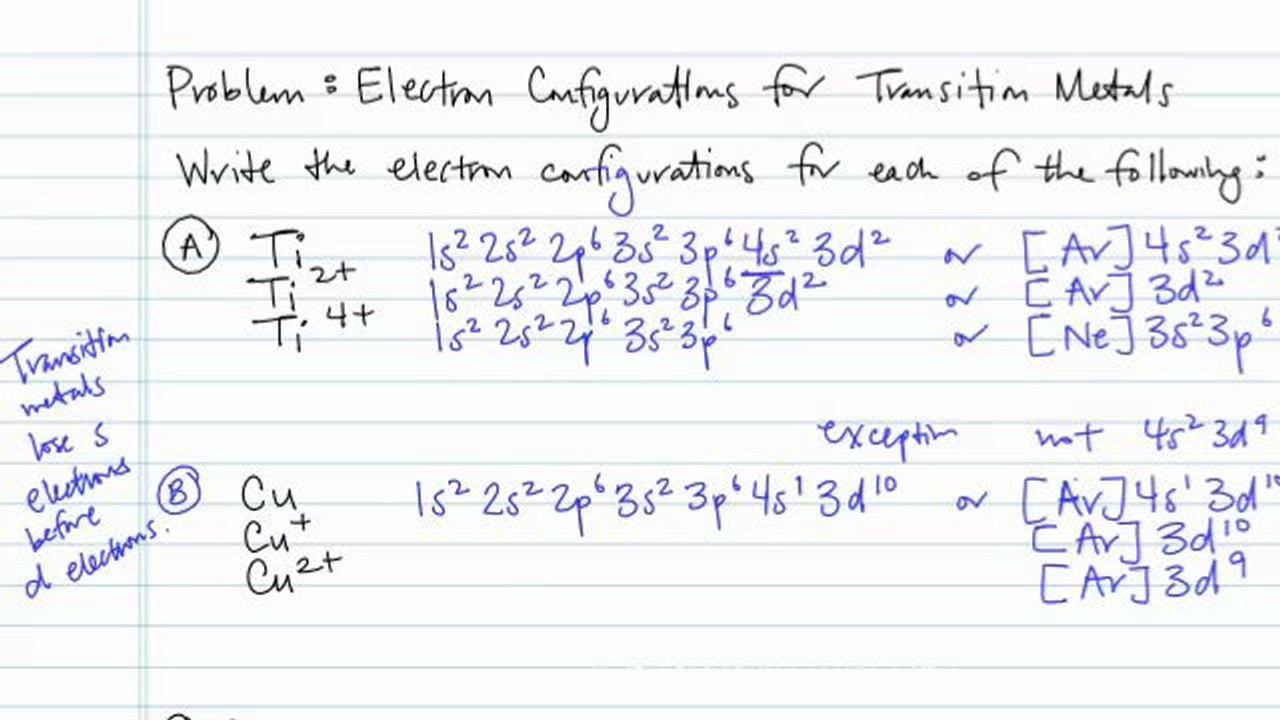
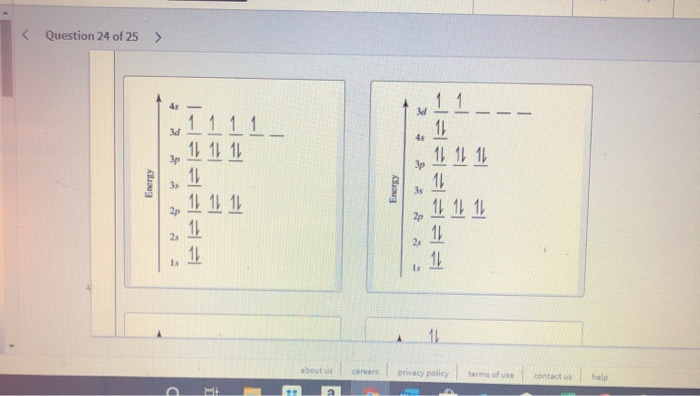
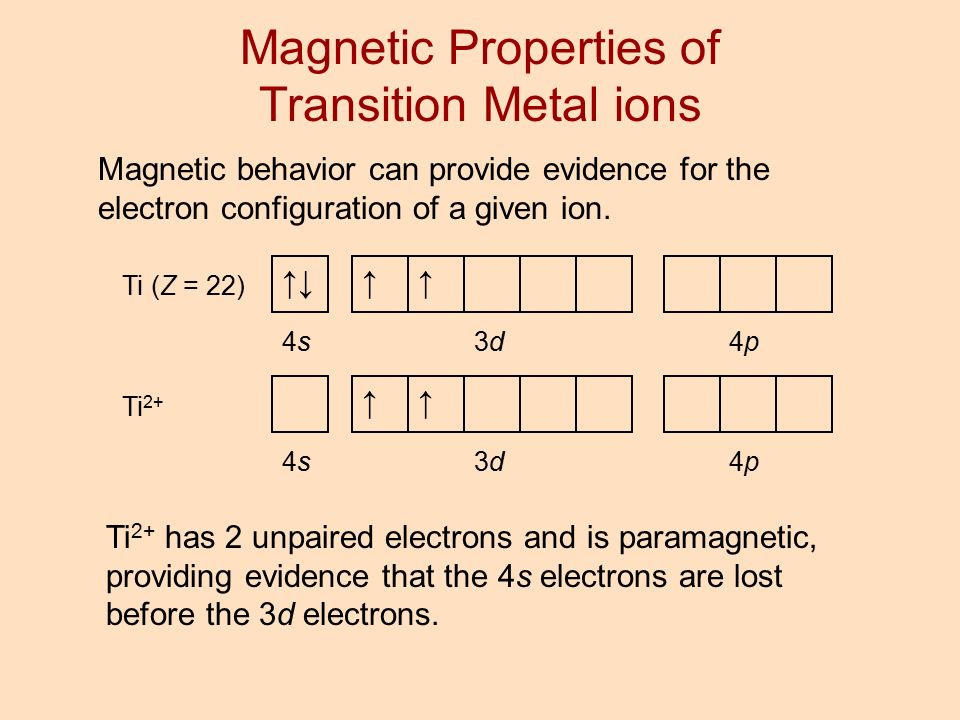
The electron configuration of a neutral titanium atom is. Ti: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2. The two electrons that are lost when the Ti2+ is formed will come from the 4s orbital, which means that the electron configuration of the cation is Ti2+:1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 silo.pub › elementary-statistics-a-step-by-stepElementary Statistics: A Step By Step Approach, (8th Edition ... Orbital period (days) Mean temperature ( F) Number of moons 3,032 7,521 7,926 4,222 88,846 74,897 31,763 30,775 1,485 36 67.2 93 141.6 483.8 890.8 1,784.8 2,793.1 3,647.2 88 224.7 365.2 687 4,331 10,747 30,589 59,800 90,588 333 867 59 85 166 220 320 330 375 0 0 1 2 63 47 27 13 1 Source: NASA. 1 Some astronomers no longer consider Pluto a planet. Learnsmart Chapter 8 Part 1 and Part 2 Flashcards - Quizlet Which of the sublevels maybe utilized in constructing a partial orbital diagram for a Period 3 element? Select all that apply.-3p-3s. Which of the following is the correct condensed electron configuration for selenium (Z = 34)? ... Ti2+ : [Ar]3d2 Mn2+ : [Ar]3d5. What is the orbital diagram for Ti 2+? I got 1s two arrows ... What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts.
Electron configuration of ti4 | electron configuration for ... This video shows how to draw the orbital diagram of titanium ti. Draw the orbital diagram for the valence shell of. Ti ti2 ti4 question. What Is The Electron Configuration Orbital Diagram And Noble Ga The colour of cations is dependent on the number of unpaired electrons present in d-orbital.
Molecular orbital diagram of TiO2, Ti2O3, TiO. Reproduced ... In the rutile IrO 2 , the site symmetry of Ir is D 2h , where the e g level is split into the a 1g and b 1g levels (σ-symmetry) and the t 2g level is split into the e g and b 2g levels (π-symmetry).
PDF 1.6 Term Symbols A brief general review of atomic ... Section 1.6 - 4 • A general term symbol that uniquely describes a specific electronic configuration looks like this: (2S+1)L J where 2S + 1 is the spin multiplicity (and S is the total spin angular momentum.) L is the total orbital angular momentum J is the total angular momentum (spin + orbital) S = 0 → "Singlet" S = ½ → "Doublet" S = 1 → "Triplet" etc.
Electron Configurations for Transition ... - Brightstorm Now if it's Ti4+, now we've taken away two additional electrons compared to the Ti2+. So we would write out 1s2, 2s2, 2p6, 3s2, 3p6, and then the 3d2-electrons would be gone. So compared to the original Titanium atom, we gave away four electrons. So both the 4s2, and the 3d2 electrons would be gone.
Orbital Diagram Of Ti2+ - Wiring Diagrams Orbital Diagram Of Ti2+. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is ...
Quantum Number Questions and Answers | Study.com The g-orbital block comes after the f-orbital block and consists of elements that have not been synthesized yet. Provide the full set of 3 quantum numbers (n, …
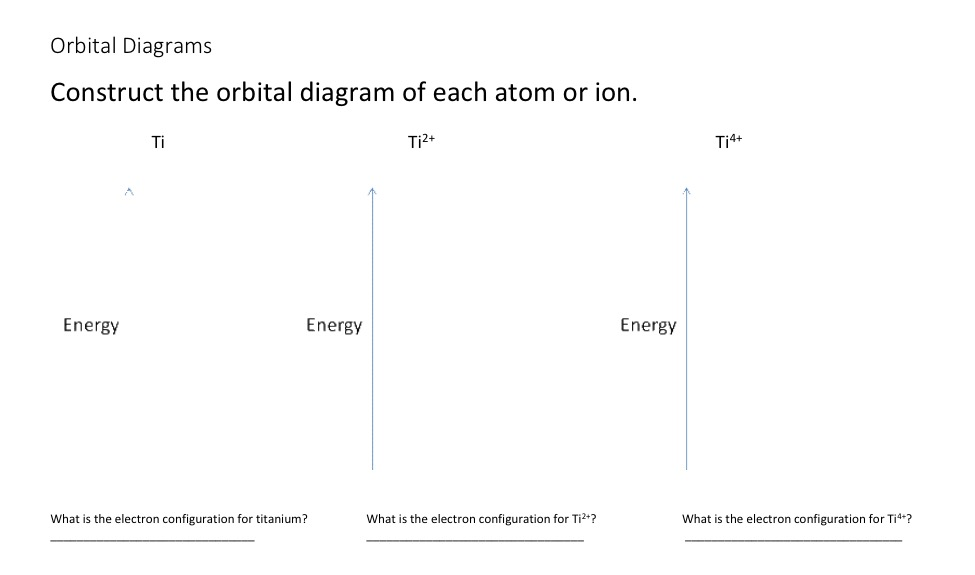
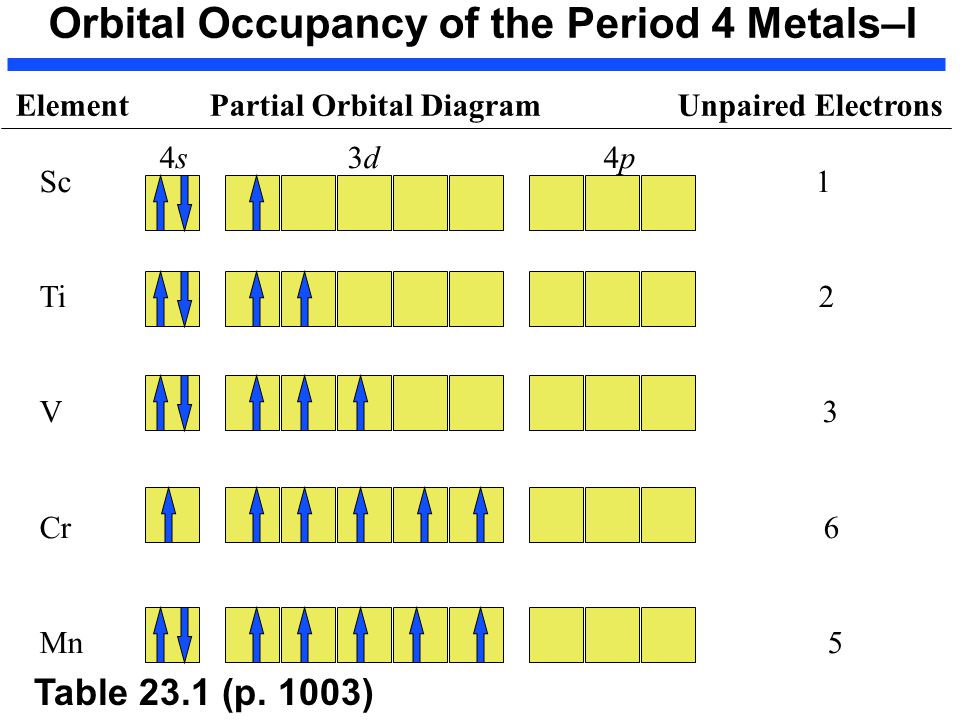
Answered: First do the electronic configuration… | bartleby Science Chemistry Q&A Library First do the electronic configuration from your periodic table. Then, from that, do the orbital diagram and dot structure. Do this for the following four substances: Mn*4 (atomic # 25), V*1 (atomic # 23), Ti2 (atomic # 22), and Co*3 (atomic # 27) First do the electronic configuration from your periodic table.
sumitai.ne.jp › urayasu浦安に住みたい!web | 市民による浦安の地域情報総合サイト 浦安の「今」を知る地域情報サイト。ほぼ毎日更新中。浦安市やその周辺地域にまつわる暮らしの情報をお届けします。
37 orbital diagram of ti - Wiring Diagram Images Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+ ... I've been tasked with drawing rhe MO diagram for Sulfure Oxide and I'm not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy ...
Konfigurasi Elektron Ion Ti3+ | ardra.biz Diagram Orbital Atom Nikel. Untuk menentukan electron yang tidak berpasangan cukup membuat diagram orbital dari subkulit yang diisi electron tidak penuh yaitu 3d yang terisi 8 elektron. Subkulit d terdiri 5 orbital yang dapat ditempati oleh 10 elektron maksimum. Jumlah elektron tidak berpasangan pada subkulit 3d dapat dilihat pada gambar berikut
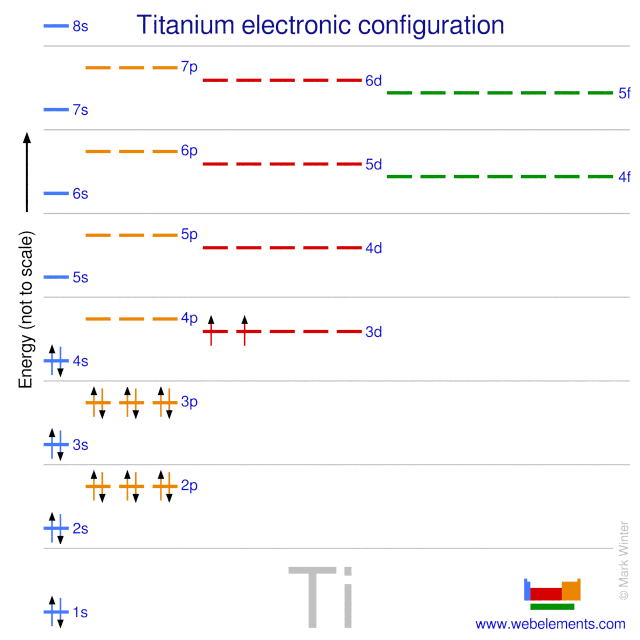
Titanium(Ti) electron configuration and orbital diagram Orbital diagram for titanium(Ti) Titanium(Ti) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2. The valency of the element is determined by electron configuration in the ...
Orbital Diagram Of Ti2+ - schematron.org Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . Which free ion has the greater number of unpaired d electrons, Ti2+ or Co2+? Draw the orbital diagram for the d orbitals in an octahedral complex containing.
WRite the electronic configuration of ti4+ ion - Brainly.in As the atomic number of Titanium is 22, it has 22 electrons. So the electronic configuration of titanium is . As is electronic configuration of Nobel gas argon, it can be represented as [Ar]. means that the titanium ion has lost four electrons and thus its electronic configuration will be [Ar].
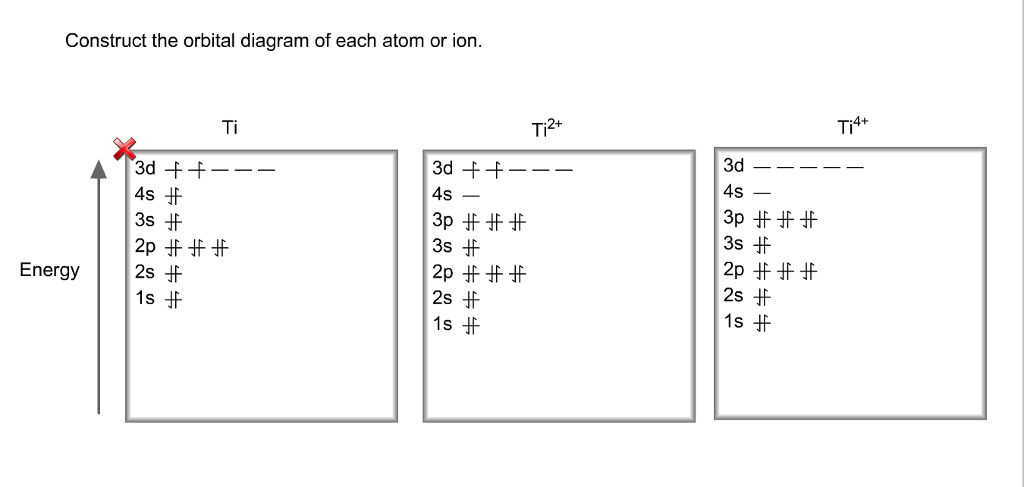
Solved Identify the orbital diagram of Ti, Ti2+, and Ti4 ... Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+.
schematron.org › comfort-zone-cz220-wiring-diagramComfort Zone Cz220 Wiring Diagram - schematron.org Nov 12, 2018 · This position is closer to where you are and reflects your comfort. Each number below corresponds to a number on the wiring diagram. I can only locate a model CZ 5) The most common method of wiring this heater is to use 1/2" EMT metal conduit and then transition over to 1/2" Flexible Metal Farenheat is a quality brand and so is Comfort Zone.
› Full_MembersFull Members | Institute Of Infectious Disease and Molecular ... Full membership to the IDM is for researchers who are fully committed to conducting their research in the IDM, preferably accommodated in the IDM complex, for 5-year terms, which are renewable.
Orbital Diagram For Ti2+ on Orbital Diagram For Ti2+. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so.
Solved Construct the orbital diagram of each atom or ... See the answer See the answer done loading. Construct the orbital diagram of each atom or ion. Ti. Ti2+. Ti4+. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Orbital Diagram of Titanium (Ti), electron configuration ... This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble...
What is the electron configuration of "Ti"^(2+)? | Socratic A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom.. In this case, titanium, #"Ti"#, is located in period 4, group 4 of the periodic table and has an atomic number of #22#. This means that a neutral titanium atom will contain #22# protons in its nucleus and #22# electrons surrounding its nucleus.
Orbital Diagram For Ti2 — UNTPIK APPS Orbital Diagram for Ti2. electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals




















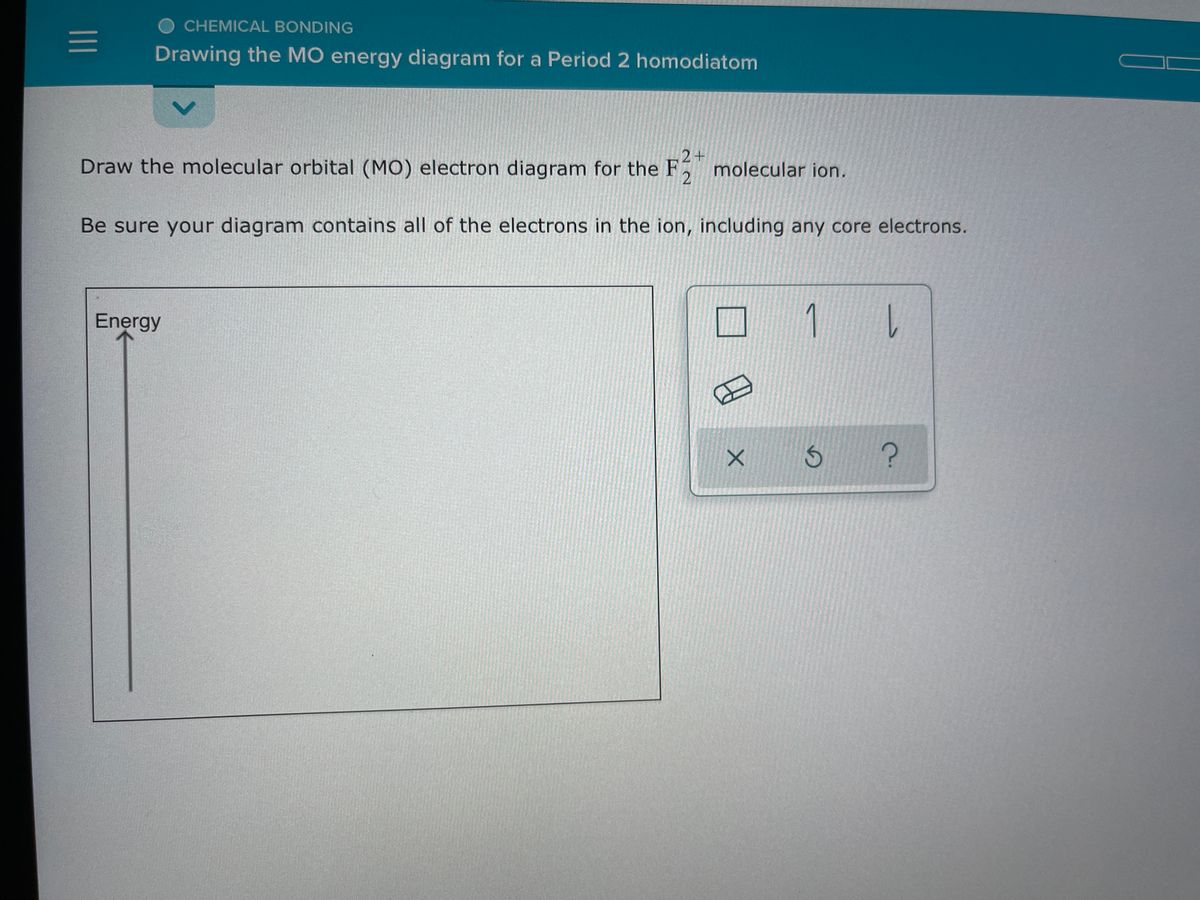



Comments
Post a Comment