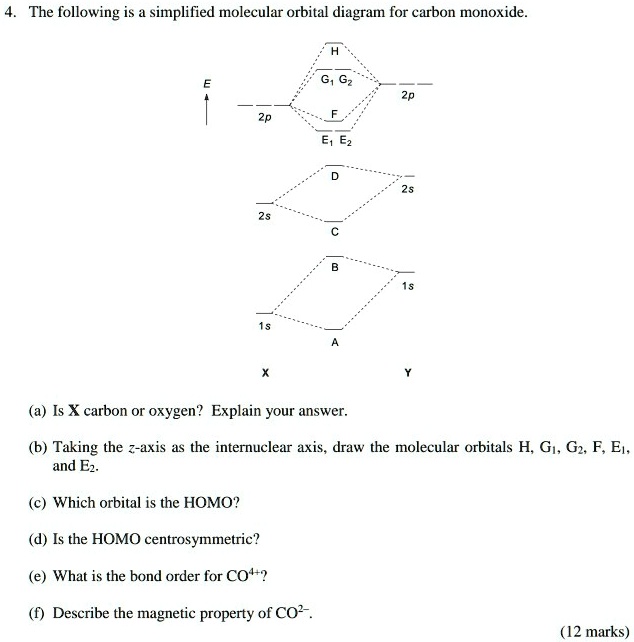
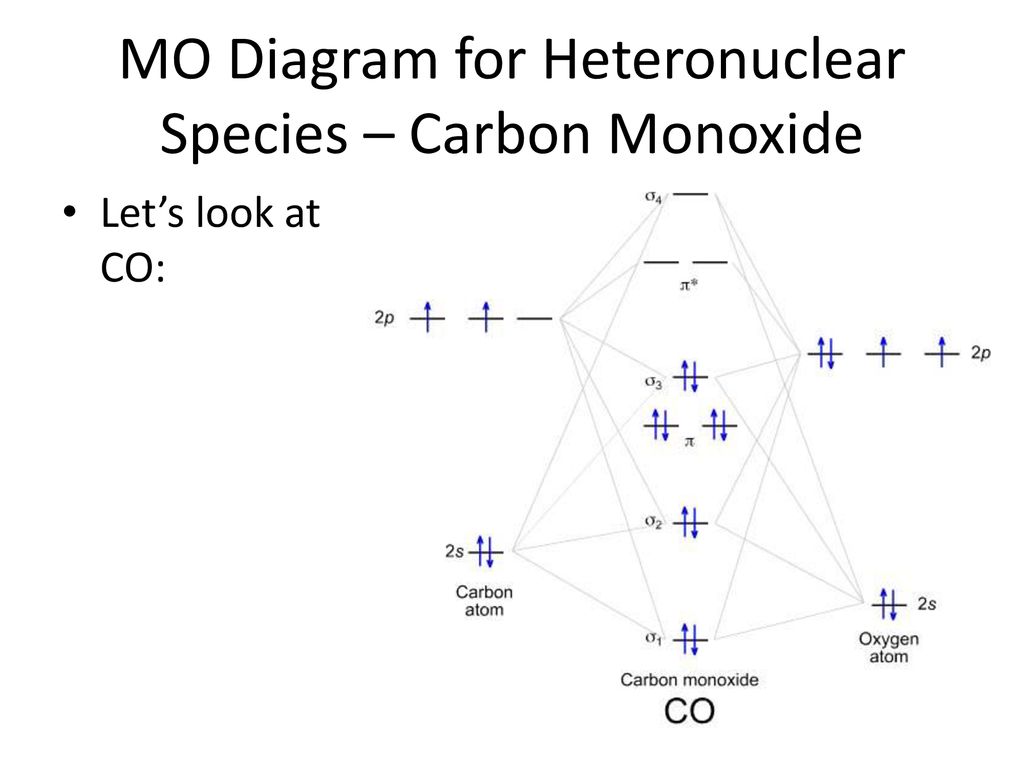
43 carbon monoxide mo diagram
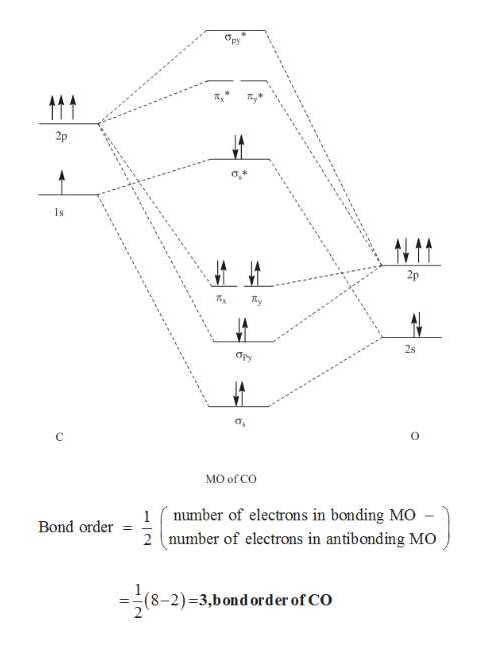
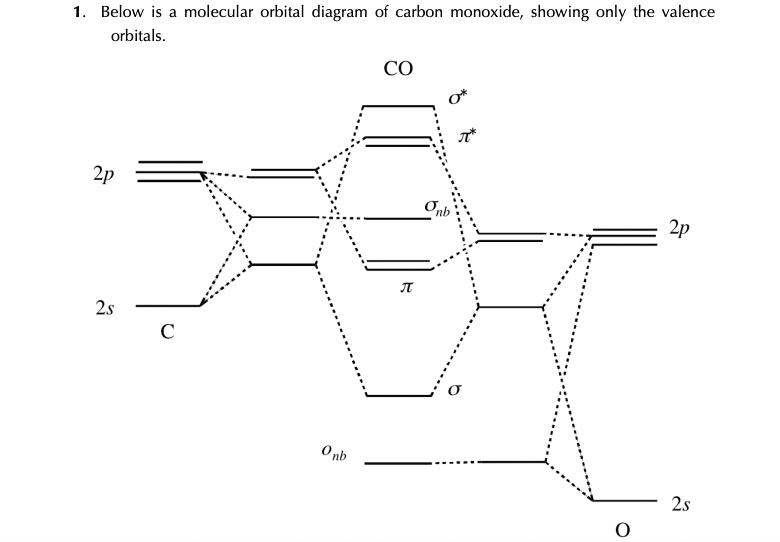
Molecular Orbital Theory The electron configuration for carbon and oxygen are: C : [He] 2s ² 2p² → 4 valence electrons; O : [He] 2s ² 2p⁴ → 6 valence electrons; There are a total of 10 electrons for carbon monoxide (CO) for which we can fill the MO diagram starting from the lowest-energy MO to the highest-energy MO. Draw MO diagram of CO and calculate its bond order ... Electronic configuration of O atom: 1s2 2s2 2p4. 2. Electronic configuration of CO molecule is: σ1s2 σ*1s2 σ2s2 σ*2s2 π2py2 π2pz2 π2px2. 3. Bond order =. = N b−N a 2 N b − N a 2 = 10−4 2 10 − 4 2 = 3. 4. Molecule has no unpaired electron, hence it is diamagnetic.
Carbon Monoxide - Facts, Bonding, Properties, Uses Molecular orbital diagram of carbon monoxide The chemical bonding in carbon monoxide is best represented by the molecular orbital diagram given below the picture, On the molecular orbital model of carbon monoxide, the two sp x hybrid orbitals of oxygen and carbon combine to give two molecular orbitals.
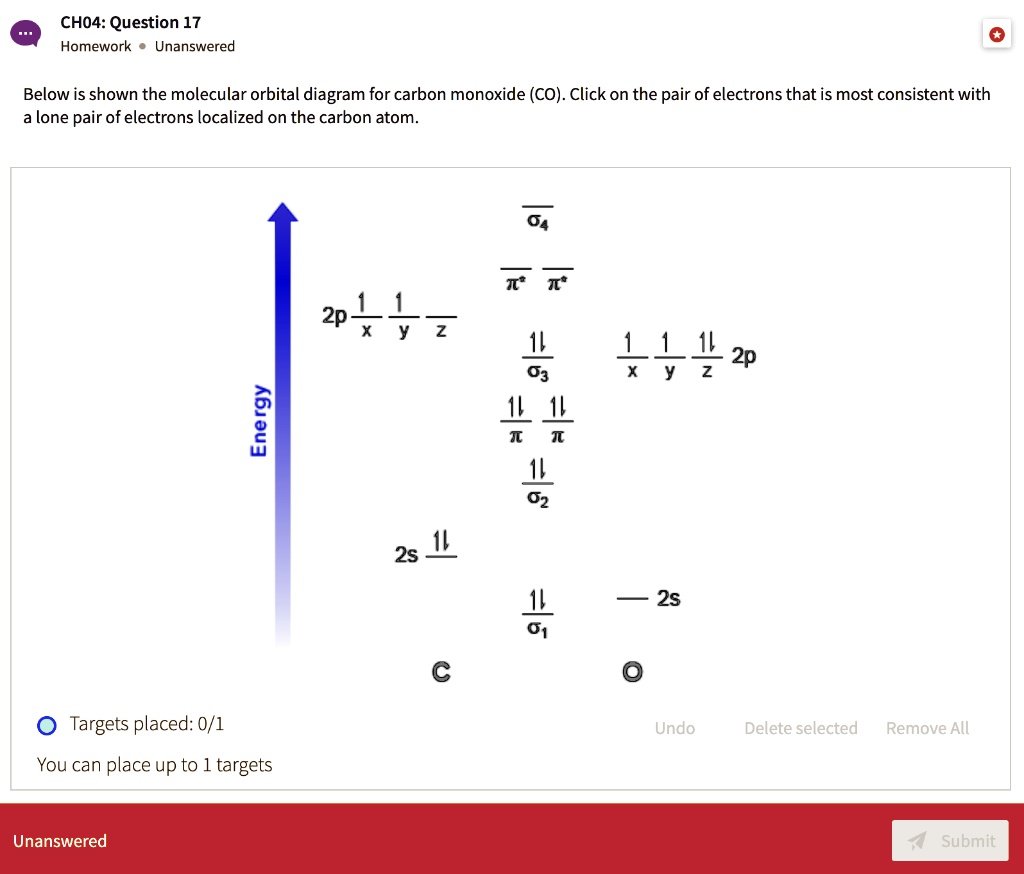
Carbon monoxide mo diagram
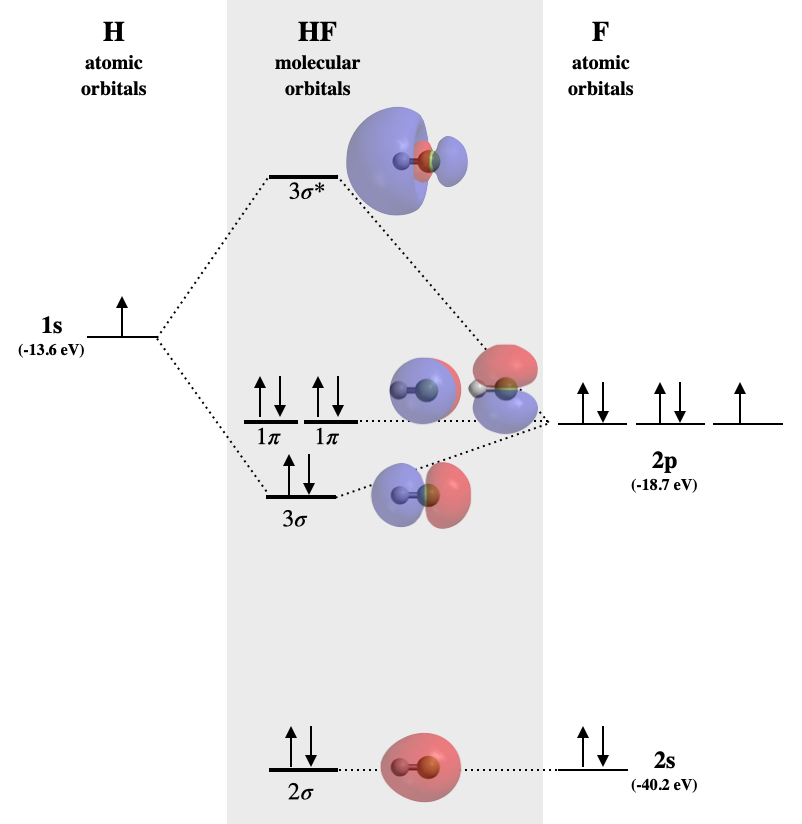
The MO Diagram of Carbon Monoxide - YouTube This webcast gives a qualitative description of constructing the MO diagram for carbon monoxide, a heterodiatomic molecule in which the AO energies are not m... What is the molecular orbital energy diagram of CO? - Quora Answer (1 of 4): PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
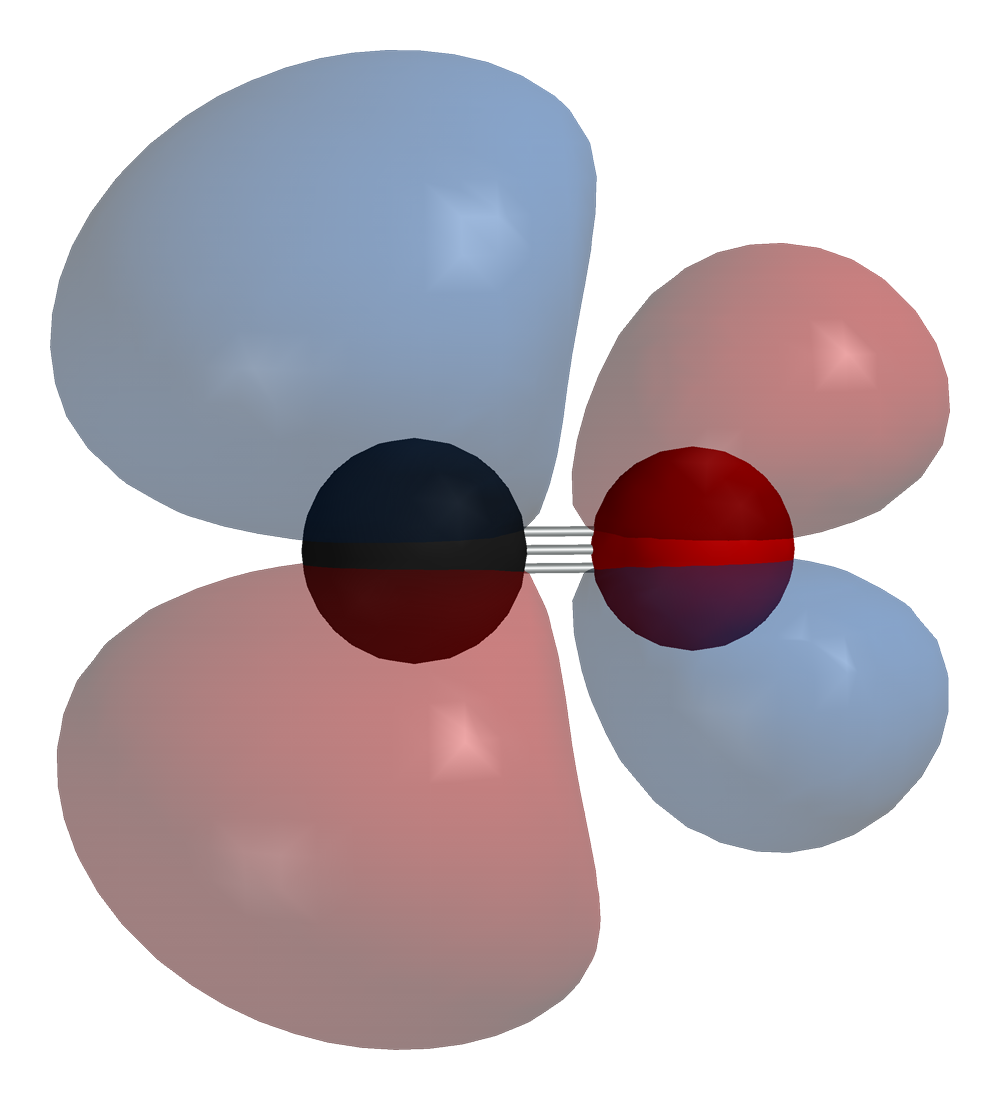
Carbon monoxide mo diagram. Carbon Monoxide Molecular Orbital Diagram Explanation generic s-p valence MO diagram for carbon monoxide CO chain one can reasonably explain, that the HOMO of carbon monoxide must be of. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not.. combinations such as CO and NO show that the 3σg MO is higher in energy. Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Interpreting the MO Diagram of Carbon Monoxide - YouTube The carbon monoxide MO isosurfaces are analyzed and assigned to the energy levels in the MO diagram. An attepmt is made to assign these levels to the electro... Carbon Monoxide Molecular Orbital Diagram Explanation There are 4 electrons in the outer shell of carbon and 6.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. MO diagram and Lewis Structure of CO - CHEMISTRY COMMUNITY MO diagram and Lewis Structure of CO. Postby y3chem » Wed Nov 06, 2013 9:21 pm. For carbon monoxide (CO) The lewis structure is :C (triple bond)O: however when I drew the MO diagram for CO it is like this: .
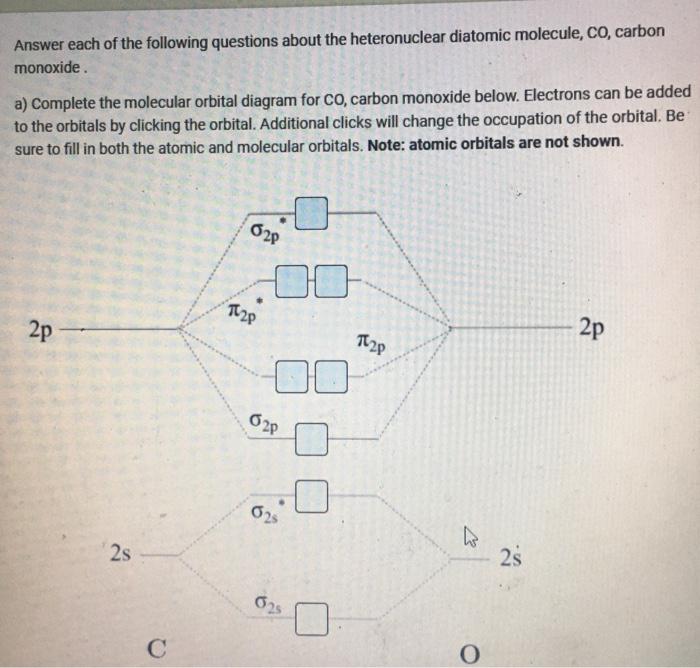
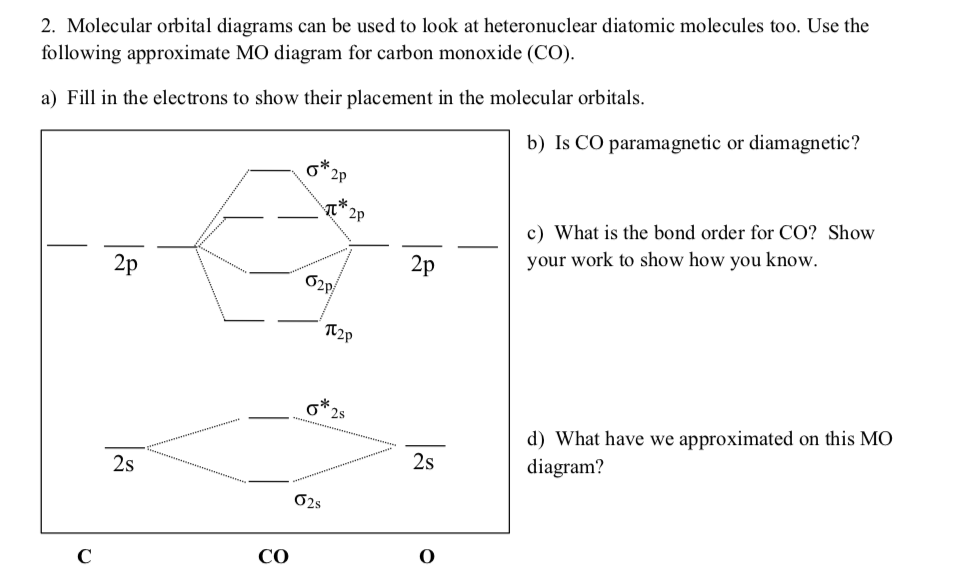
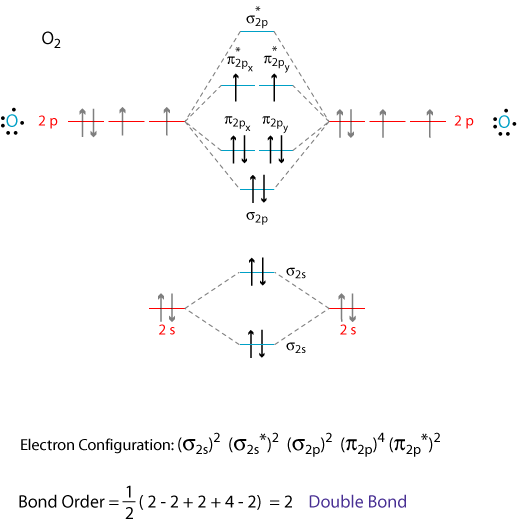
What is the molecular orbital energy diagram of CO? - Quora Jan 7, 2017 — As we know across the period the nuclear charge increases, The oxygen has a greater effective charge than carbon. Therefore bonding MO has more of oxygen ...4 answers · 29 votes: First let us know what molecular orbital diagram is: A molecular orbital diagram, or MO diagram, ...What is the bond order of CO? - QuoraSep 16, 2016Why in the MOT diagram of CO, 2p sigma has higher energy ...Sep 8, 2020How does carbon monoxide act as an electron donor through ...Nov 22, 2017What do the molecular orbitals of cyanide look like, compared ...Oct 9, 2014More results from NOTES-The_MO_Diagrams_of_Carbon_Monoxide - The MO Diagram ... The MO Diagram of Carbon Monoxide Oxygen Carbon 2p x 2p x 2p y 2p y 2p z 2p z 2s 2s σ s σ s * σ p σ p * π y * π z * π y π z C O Begin just as with F 2. Compare the AO energies for CO vs. F 2. Can we ignore the C2s / O2p x interaction? PDF EXAMPLE #2: Carbon Monoxide, CO - University of Guelph CHEM 2060 Lecture 29: Heteronuclear Diatomics MO L29-1! EXAMPLE #2: Carbon Monoxide, CO Recall: The MO energy level diagram for O 2 is not the same as the MO energy level diagram for the C 2 gas phase fragment. How do we create an MO energy level diagram for a heteronuclear diatomic species in which both atoms have valence s and p orbitals? N2+ Mo Diagram - schematron.org The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.
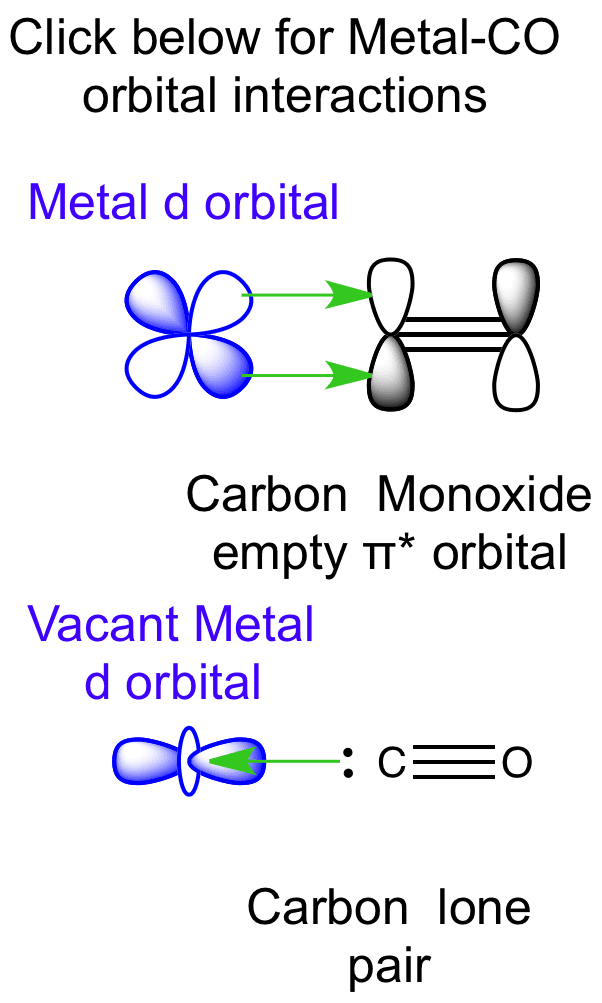
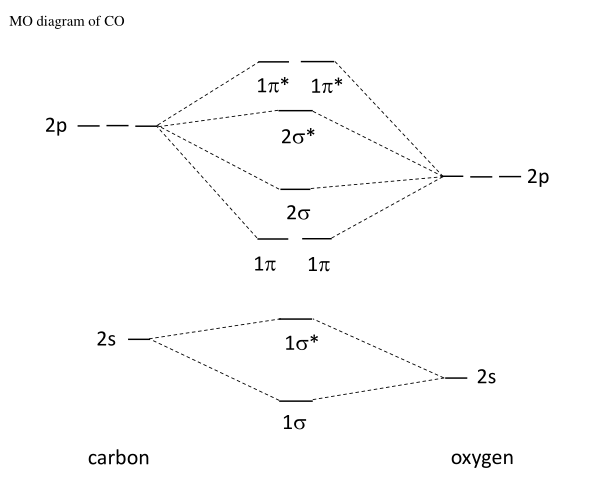
How to rationalise with MO theory that CO is a two-electron ... May 16, 2016 — The key to explaining that carbon monoxide is a two electron donor through carbon is to start with with a very fundamental MO scheme. This is ...2 answers · Top answer: I had to give this a good, long thought, after I initially thought this is a question ... Carbon monoxide | CO - PubChem It is a one- carbon compound, a gas molecular entity and a carbon oxide. It is a conjugate base of a carbon monoxide (1+). Carbon Monoxide is an odorless, tasteless, poisonous gas, CO, that results from the incomplete combustion of carbon. Inhalation causes central nervous system damage and asphyxiation. (left) Simplified MO diagram of CO with electronic ... The use of Carbon Monoxide (CO) as a therapeutic agent has already been tested in human clinical trials. Pre-clinically, CO gas administration proved beneficial in animal models of various human ... 5.3.1: Polar bonds - Chemistry ... - Chemistry LibreTexts Carbon monoxide MO diagram Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The valence molecular orbitals in both atoms are the 2 s and 2 p orbitals. The molecular orbital diagram for carbon monoxide (Figure 5.3.1.
Molecular Orbitals for Carbon Monoxide The HOMO of carbon monoxide is σC(2p)O(2p) because the antibonding contribution from sp mixing pushes it above the π-bonding orbitals in energy Its main components are C 2 s and C 2 p z , so it is strongly polarised towards carbon, and will bond to σ -acceptor species through carbon, providing that the CO ligand is also acting as a π -acceptor (the 'synergic effect')
What is molecular orbital diagram of CO? - handlebar ... Carbon monoxide MO diagram. Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The valence molecular orbitals in both atoms are the 2s and 2p orbitals. The molecular orbital diagram for carbon monoxide (Figure 5.3.
Carbon Oxides - University of Illinois Urbana-Champaign The molecular orbital diagram of carbon monoxideis very similar to that of molecular nitrogen. Carbon, with 4 valence electrons, and oxygen with 6 valence electrons, together have the same number of electrons as dinitrogen. Oxygen is more electronegative than carbon, so its orbitals are more highly stabilized and lower in energy than the carbon orbitals.
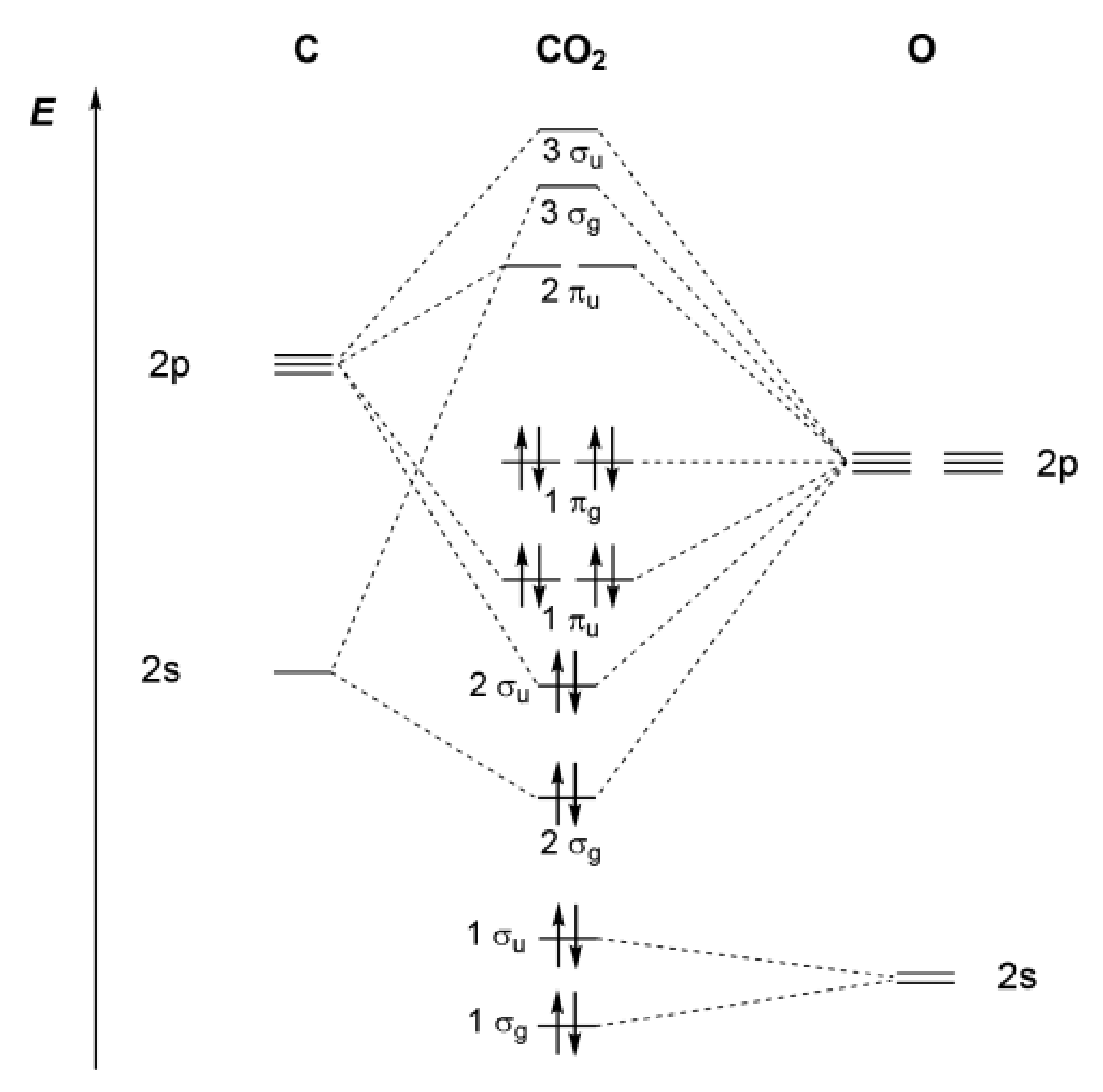
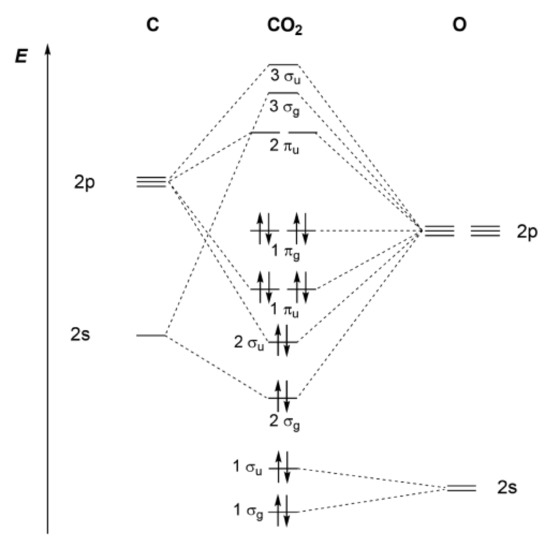
PDF MO Diagrams for Linear and Bent Molecules Carbon Dioxide by Reducible Representations Γ2s= Ag+ B1u Γ2pz= Ag+ B1u Γ2px= B2g+ B3u Γ2py= B3g+ B2u B3u B2u Ag Ag 2p x 2p y 2p z 2s B2g B3g B1u B1u These are the same group orbital symmetries that we got using inspection. We can (re)draw them. 5. Find matching orbitals on central atom Ag B1u B3u B2u 6. Build MO diagram…
Carbon monoxide - Wikipedia Carbon monoxide is also a byproduct of the reduction of metal oxide ores with carbon, shown in a simplified form as follows: MO + C → M + CO. Carbon monoxide is also produced by the direct oxidation of carbon in a limited supply of oxygen or air. 2 C(s) + O 2 → 2 CO(g)
Bonding in Metal Carbonyls: Explaination, Type, Property ... Molecular Orbital Diagram of CO To understand the bonding in metal carbonyls, we need to first learn the Molecular Orbital ( M O) diagram of carbon monoxide. There are ten electrons in the carbon monoxide ligand. The order of energy of the molecular orbitals and the accommodation of ten electrons of the carbon monoxide can be shown as:
CO Lewis Structure, Geometry, and Hybridization ... Below mentioned are the steps to draw Lewis structure of Carbon Monoxide: Find total valence electrons: It is 10 to form the carbon monoxide. Find how many electrons are needed: It is 6 for one carbon monoxide (CO) molecule as per the octet rule. Look for the total number of bonds forming: Triple covalent bonds are forming in one carbon monoxide (CO) molecule; Choose a central atom: Both the atoms will be central; Draw the lewis diagram
Neatly hand-draw the MO diagram for carbon monoxide ... Neatly hand-draw the MO diagram for carbon monoxide. Then, under that drawing, discuss... 1. where the electrons are thought to come from this diagram when donating to make a M-C bond. 2. what molecular orbital receives electron density from backbonding. Question: Neatly hand-draw the MO diagram for carbon monoxide.
Molecular orbitals in Carbon Monoxide - ChemTube3D Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. Hydrogen | Fluorine | Nitrogen | Hydrogen Fluoride | Carbon Monoxide | Methane | Ammonia | Ethylene | Acetylene | Allene | Formaldehyde | Benzene
What is the bond order of CO? - Pursuantmedia.com Carbon monoxide MO diagram. Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The valence molecular orbitals in both atoms are the 2s and 2p orbitals.
PDF BONDING IN METALLIC CARBONYLS Carbon monoxide In order to understand the bonding in metal carbonyls, let us first see the MO diagram of carbon monoxide. Figure: Molecular Orbital Energy Level Diagram of Carbon Monoxide . The order of energy of the molecular orbitals and the accommodation of ten electrons of the
bond - How can the dipole moment of carbon monoxide be ... The MO diagram shows that the $\ce{CO}$ molecule forms three filled MO's with σ symmetry and two MO's with π symmetry. Of the five filled MO's (10 electrons) formed for $\ce{CO}$, only four of them can be half-filled from carbon electrons (4 valence electrons).
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
What is the molecular orbital energy diagram of CO? - Quora Answer (1 of 4):
The MO Diagram of Carbon Monoxide - YouTube This webcast gives a qualitative description of constructing the MO diagram for carbon monoxide, a heterodiatomic molecule in which the AO energies are not m...
















Comments
Post a Comment