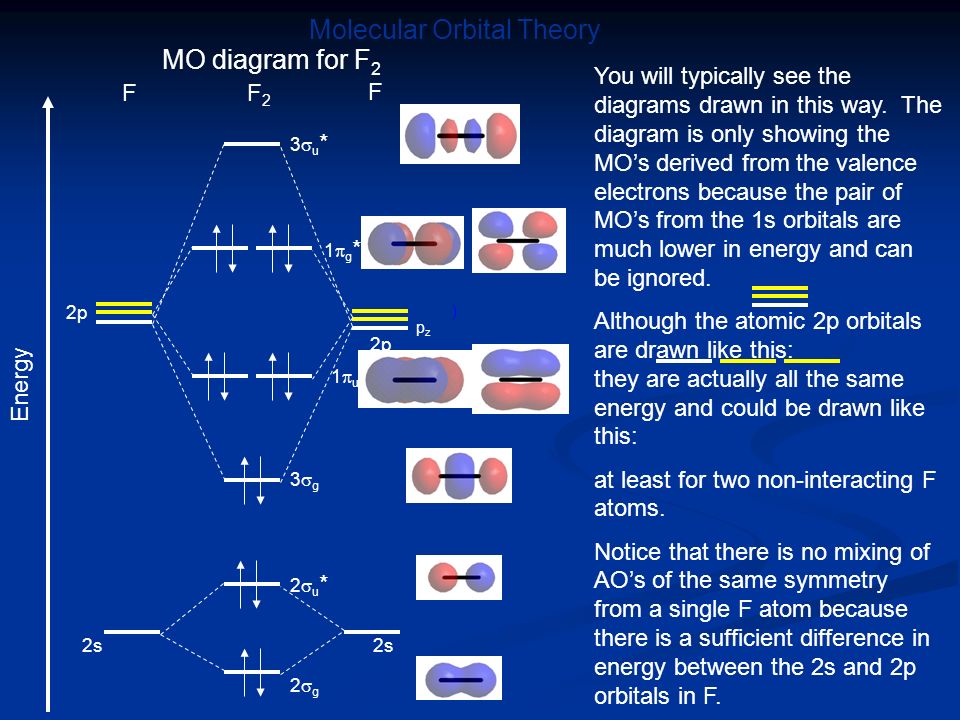
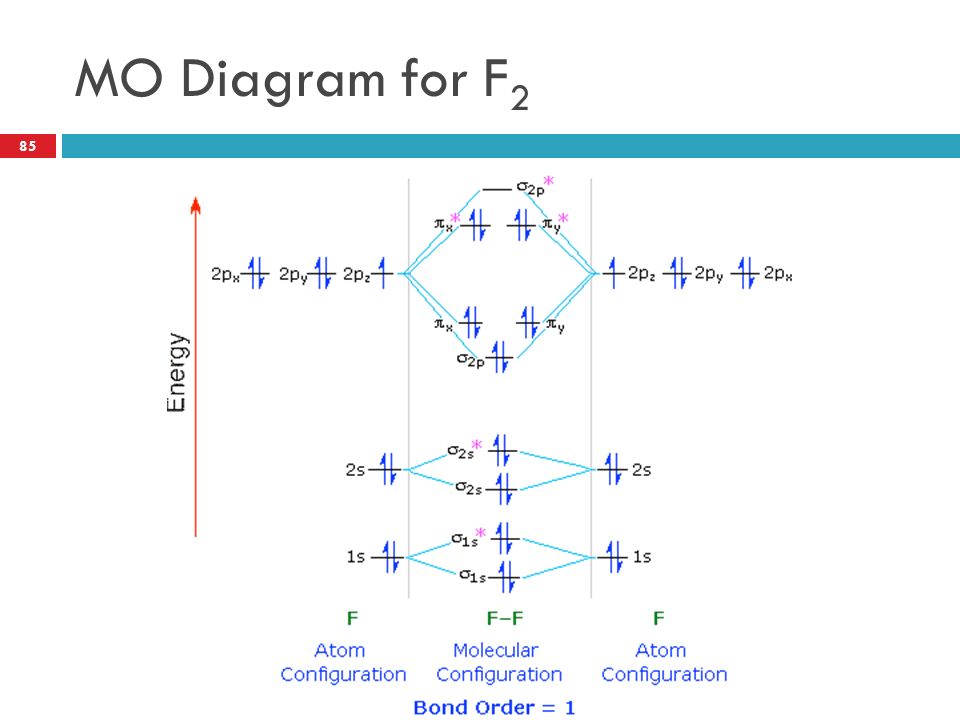
42 mo diagram of f2
If you draw the molecular orbital diagram for F2, you can see that the highest energy electrons are in pi anti-bonding orbitals. Removing one of these is relatively easier since the orbital is anti-bonding, or higher energy than a normal orbital in F. On the other hand, fluorine is one of the hardest elements to remove an electron from because ... Co‐degradation of interferon signaling factor DDX3 by PB1‐F2 as a basis for high virulence of 1918 pandemic influenza.
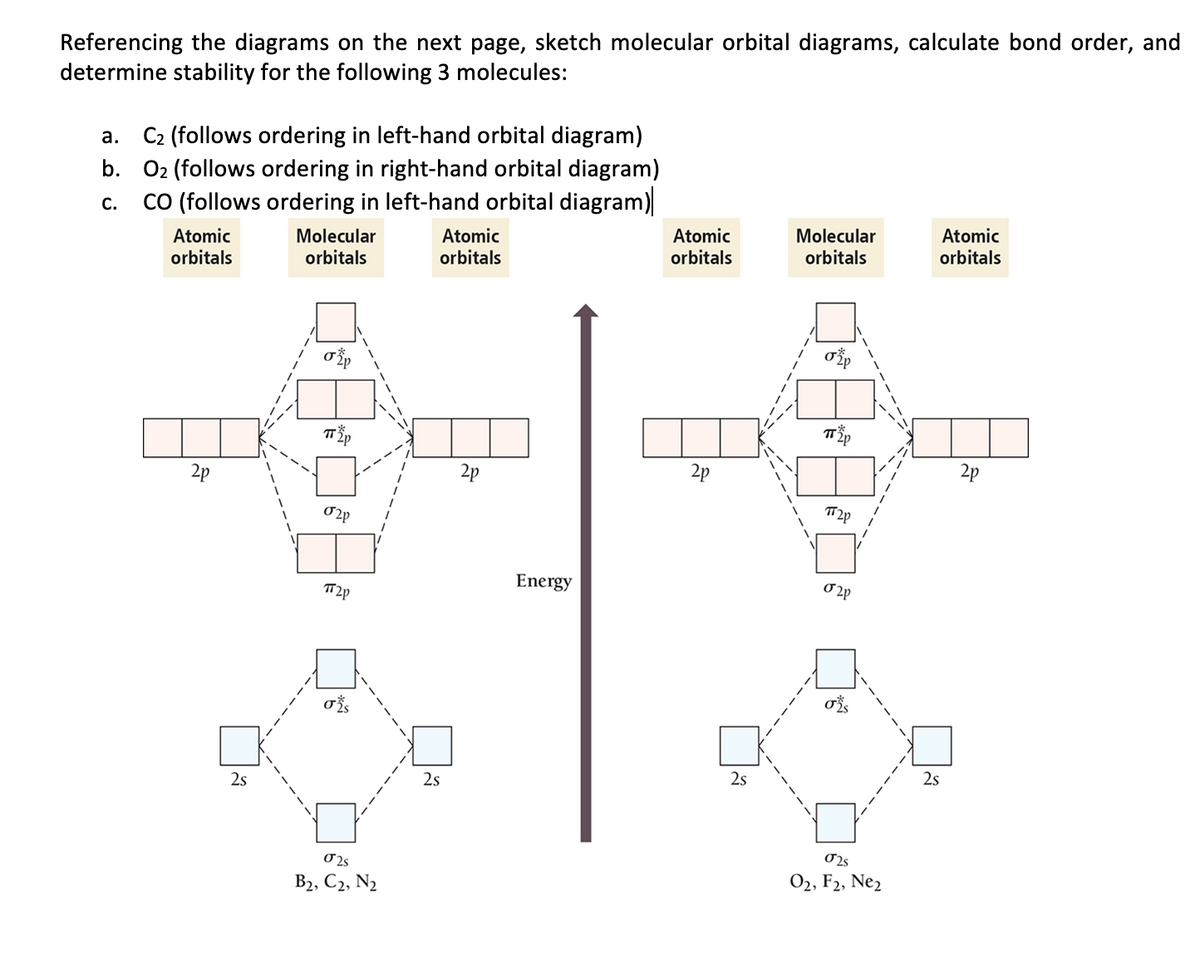
Setting up the diagram Start by considering the axial definition: Always put the z-axis along the bond in diatomics. Always add a diagram clearly showing how the axial system related to your molecule on your MO diagram then start the diagram itself, remember the vertical "axis" of the whole diagram is energyand the horizontal axis are

Mo diagram of f2
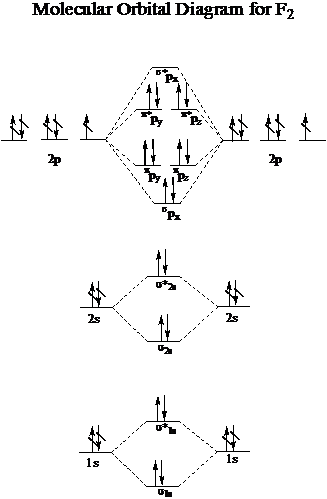
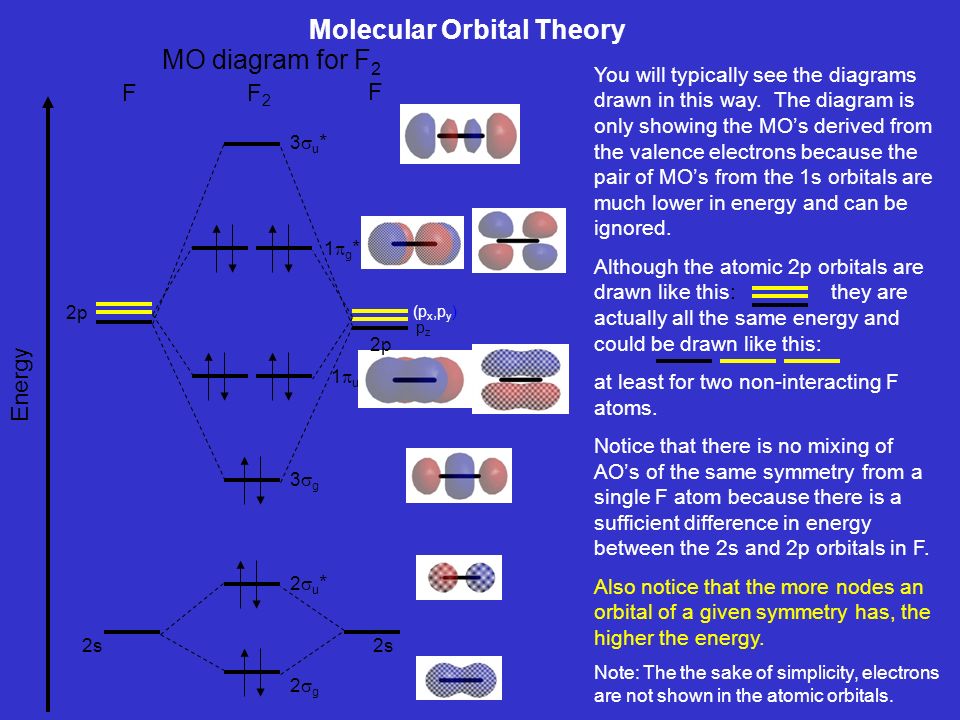
Answer (1 of 3): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ... Jul 03, 2017 · For example, an ns/ns overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this: and an np/np overlap for O2 and F2 gives: So, the full MO diagram is: Thus, the valence electron configuration is: (σ2s)2(σ* 2s)2(σ2pz)2(π2px)2(π2py)2(π* 2px)2(π* 2py)2. Answer link. As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond.
Mo diagram of f2. Draw an MO diagram for the valence electrons in F2. What is the bond order? How many o and π bonds are there? What is the HOMO and LUMO? What is the magnetism of the species? FREE Expert Solution 83% (476 ratings) Sign up for free to view this solution Sign up for free. 572,423. students enrolled. 97%. ... Identify the MO diagram for F2. F2 valence e−: diagram A 6 diagram A 8 diagram A 10 diagram B 12 diagram B 14 Solution In B2, C2, and N2, the 𝜎2𝑝 orbital is higher in energy than the 𝜋2𝑝 orbitals as shown in diagram A. Here is a video that discusses over the Molecular Orbital Diagram for F2+ and F2+. Then compare their bond length, strength, bond order etc. And explaining a... #3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron.
Dot Da Genius DrumkitSeamlessR - Modular Aggression Pegboard Nerds - End Is Near Sample Pack The Fantastic Sounds of Jay Dee AKA J Dilla The Fantastic Sounds of Jay Dee AKA J Dilla The Fantastic Sounds of Jay Dee AKA J Dilla KJ SAWKA 40x40x40 DRUM PACK Transcendent Trap 2 by Paradigm Theorem Dot Da Genius Drumkit Jordy Dazz... 방법 ) [ MO Diagrams of B2, C2, N2 ] [ MO Diagrams of O2, F2, Ne2 ] 결합성 MO에 있는 전자 수 = 반결합성 MO에 있는 전자 수 이므로, 즉 결합 차수 = 0 이므로, Ne2 이원자분자는 자연계에 존재하지 않는다. [ 목차 ] 제9장 분자의 기하학적 구조와 결합 이론 [키워드] MOT 기준문서, 분자오비탈이론 기준문서, 분자오비탈 기준문서 Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F. ... Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine Primary Citation of Related Structures:An inhibitor of alpha-thrombin was designed on the basis of the X-ray crystal structures of thrombin and trypsin. The design strategy employed the geometric and electrostatic differences between the specificity pockets of the two enzymes. These differences arise due to the replacement of... Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading. The Lewis theory of chemical bonding helps us visualize the arrangement of atoms—how they are attached or bonded—in molecules. The valence electrons in each atom are the ones that participate in the bonding, and hence they are the only ones displayed in the Lewis structures. It is to be noted though that this theory about the electronic structure is quite primitive and most limited. In a typical Lewis structure, each valence electron is represented as a dot, and a covalent bond between two atoms (formed as a result of sharing of two electrons) is represented as a line. Several atoms tend to seek eight electrons in their valence shell through chemical bonding; this is referred to as the octet rule and is reflected in the Lewis structure of a molecule. Hydrogen is an exception, though; it seeks a duplet, not octet, because it has only one electron in its K shell, and thus needs only one more to achieve the maximum capacity of K shell. Noble gases already have completely filled valance...
The Best and Completed Full Edition of Diagram Database Website You Can Find in The Internet ... 108 Schematic Diagram Free Download Vw Beetle Wiring Diagram Diagram Order Diagram For 2 Way Light Switch Alarm Wiring Diagram Toyota Prius Wiring Diagram Wiring Diagram With Points Outboard Wiring Harness Diagram Alternator Wiring Diagram Corvette Engine Diagram G6 Stereo Wiring Diagram Stuff Led Wiring Diagram Ford Ranger Engine Computer Diagram Stop Relay Wiring Diagram Tahoe Engine Diagram Motorcycles Wiring Diagrams Diagram For 1998 Dodge Ram 3500 4x4 Cell Wiring Diagram Chrysler Imperial W
The Best and Completed Full Edition of Diagram Database Website You Can Find in The Internet ... Post Wiring Diagram Pajero Electrical Wiring Diagrams 2001 2003 Nissan Xterra Wiring Diagram Kenworth Wiring Diagram Fuse Box Diagram Motor Toyota 5e Ke Wiring Diagram Audi Tt Wiring Diagram 450sl Power Switch Wiring Diagrams 1975 Diagram M5 Fuse Diagram Controlled Lighting Wiring Diagram Yy150t 12 Wire Diagram Ford Explorer 4x4 Engine Diagram For Equivalence Of Tenths And Hundredths Tail Light Wiring Diagram Dual Filiment Bay Ceiling Fan Pull Switches Wiring Diagram Flow Diagram For Banking Sys
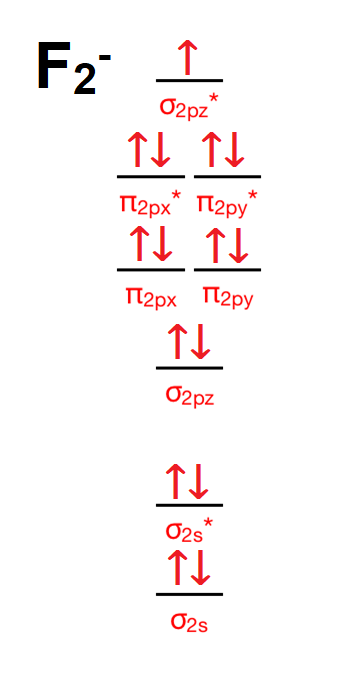
Valence electrons in F2- = 15 It's molecular orbital confirguation will be Bond order = (Bonding electron - antibonding electron)/2 = 8-7/2 = 0.5 bond order No of sig …. View the full answer. Transcribed image text: 2. Draw an MO diagram for the valence electrons in the F2 ion. What is the bond order?
Hey there! We have to draw a molecular orbital diagram for the F 2-ion, determine bond order and decide whether the molecule is diamagnetic or paramagnetic.. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule.
The Best and Completed Full Edition of Diagram Database Website You Can Find in The Internet ... Command Wiring Diagrams Diagram Of Steps Nissan Xterra Fuse Box Diagram Wiring Schematic Gr Sx25ek Gr Sx25ex Compact Vhs Camcorder Schematic Diagram Manual Acclaim Radio Wiring Diagram Electrical Wiring Diagrams Wiring Diagram Scout Engine Wiring Diagram Chevy Venture Fuse Box Diagram Diagram 1995 Ford Econoline Tunes Wiring Diagram Kia Rio Wiring Diagram Trailer Plugs Wiring Diagram 6 Spade Line Wiring Diagram Dash Fuse Diagram On 97 Nissan Maxima Master Wiring Diagram Usuario Skyhawk Fuse Box
brings two electrons to the molecular orbitals. There would be four electrons to fill into our molecular orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO. What we gain in the bonding sigma MO, we lose in the anti-bonding sigma-star MO. There is no
com/3047 ] MO Diagrams of O2, F2, Ne2 [ 관련 글 https://ywpop.tistory.com/5000 ] N2와 N2^+의 질소-질소 결합길이. [키워드] 분자오비탈, MO Diagrams of C2, MO Diagrams of N2, MO of B2, MO of C2, MO of N2, B2의 MO 기준문서, C2의 MO 기준문서, N2의 MO 기준문서 MO Diagrams of B2, C2, N2.hwp 반응형 좋아요 5 공유하기 글 요소 구독하기 좋은 습관... 화학 MO Diagrams of B2, C2, N2 by 영원파란 2016. 5. 25. 카카오스토리...
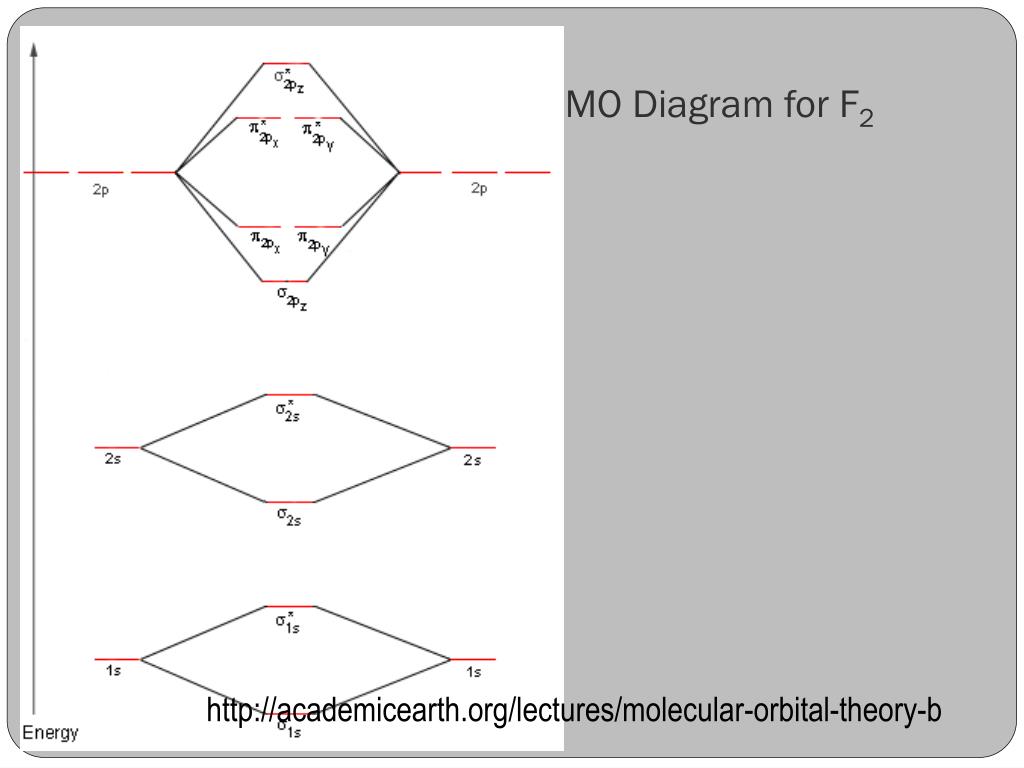
MO Diagrams of O2, F2, Ne2 MO diagrams for diatomic molecules 결합 차수 = 1/2 (결합성 MO 전자 수 – 반결합성 MO 전자 수) O2의 결합 차수 = (6 – 2) / 2 = 4/2 = 2 F2의 결합 차수 = (6 – 4) / 2 = 1 Ne..
The molecular orbital diagrams of , and are drawn in the attached image. There is no unpaired electron present in the MO diagram of and all the electrons are paired up so it is diamagnetic in nature. There is one unpaired electron present in the MO diagram of and therefore it is paramagnetic in nature.
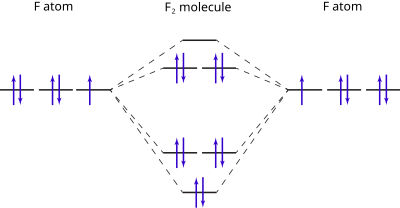
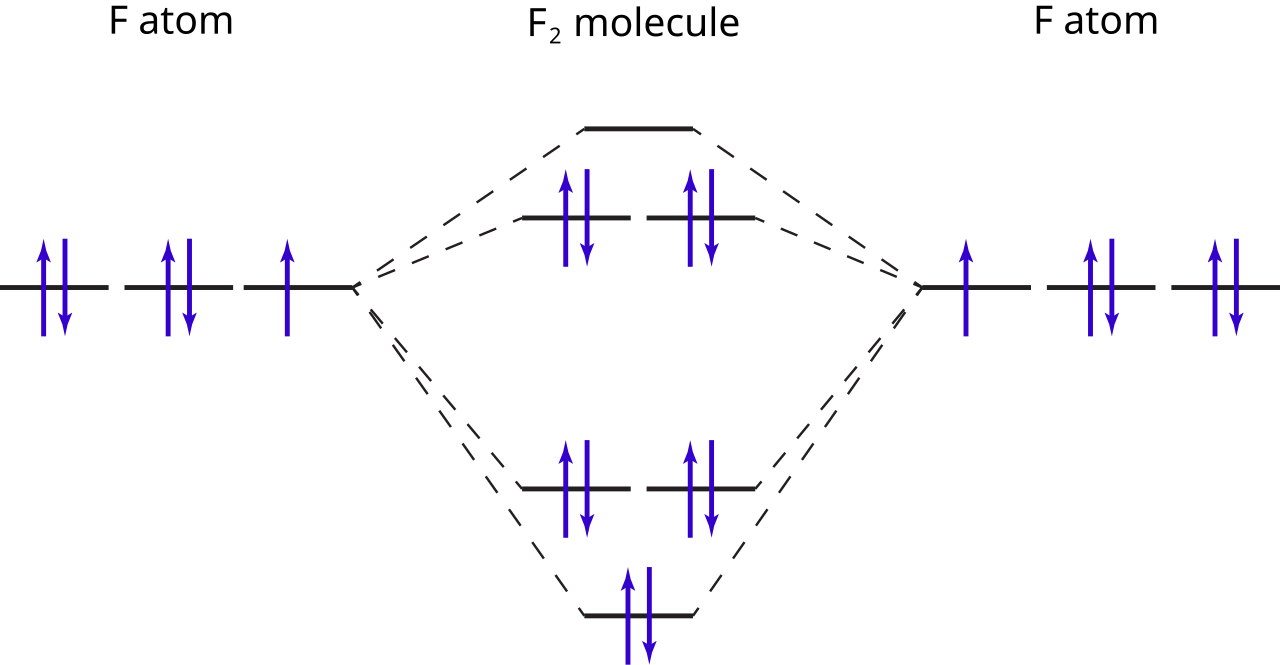
Molecular orbital diagram and bond order of fluorine molecule . Fluorine molecule is formed by the combination of atomic orbitals of two fluorine atoms, each having nine electrons, thus making 18 electrons.; These 18 electrons are filled in various molecular orbitals, in the increasing order of their energies (aufbau principle) and on the basis of Hund's rule and Pauli's exclusion principle as ...
Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
Mo diagram for F2 -.jpg -. School Uni. Tartu. Course Title CHEMISTRY 123. Uploaded By Vudef. Pages 1. This preview shows page 1 out of 1 page. View full document. End of preview. Want to read the entire page?
An extension of Mo Kβ XES to nitrogenase‐relevant model complexes shows that the method is sufficiently sensitive to act as a spectator probe for redox events... A representative energy level diagram is displayed below the emission spectrum in Figure 2 C. Figure 2 C shows the non‐resonant emission processes that result... Conclusion A systematic experimental and theoretical study of Mo Kβ XES has been performed for a range of...
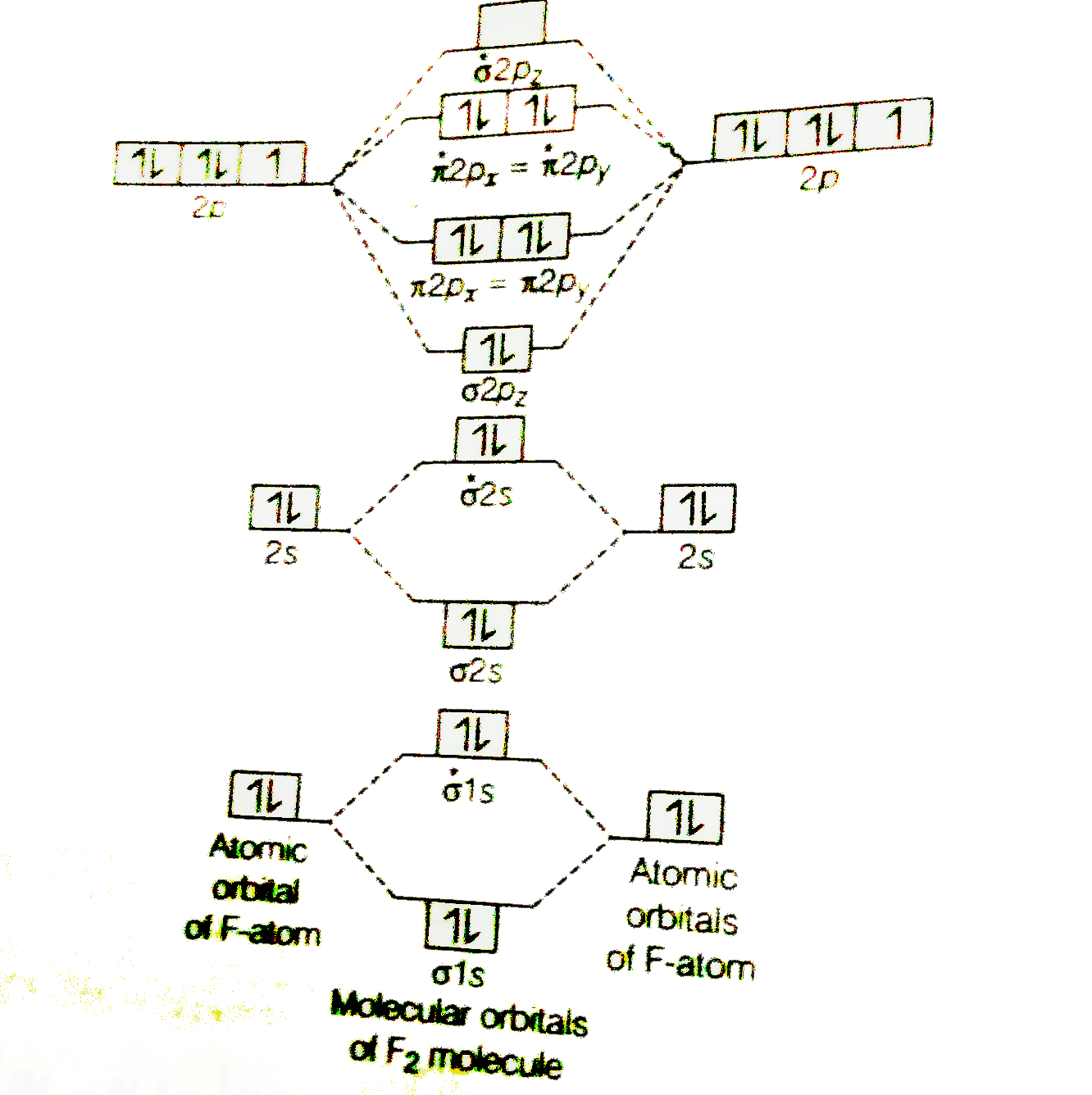
When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x 2, π2p y 2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
This video is about MO Diagram #2 - F2
Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following: Open in App. Solution. Verified by Toppr. Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems >
Synthesis and Characterization of Low-Dimensional Chalcogenide Compound via a Molten Salt Method 덕수, 최;혜식, 윤;화숙, 오;돈, 김;호섭, 윤;윤봉, 박 Journal of the Korean Chemical Society. 2004. Oct, 48(5): 504-509 DOI : http://dx.doi.org/10.5012/jkcs.2004.... Calculated and Observed X-ray Powder Diffraction Pattern of KCu4Se3 PPT Slide Lager Image Calculated and Observed X-ray Powder... Advanced Home Journals Publish Aids About Top Abstract...
The reaction of Cu metal with mixed alkali metal polyselenide flux (KNaSe x ) produced large plate-like crystals of KCu 4 Se 3 . The structure of KCu 4 Se 3 , determined with X-ray single crystal diffraction techniques, is tetragonal (P4/mmm, a=4.013(1))\AA , c=9.712(1))\AA , z=1, R=6.7%). The structure is composed [Cu 4 Se 3 ] n− n double layers which are made of...
(1) Sketch an MO energy diagram showing only the molecular orvitals and electiron distribution in CO. Label the energy levels according to the type of orbitals from which they are made, whether they..
Finally, we have the 2px + 2px σ* (sigma star) antibonding MO. The diagram of the bonding and antibonding MOs is shown below: Electrons are added to the MOs (bonding and antibonding) using the same rules that are used for adding electrons to atomic orbitals: The aufbau principle, lowest energy MOs fill first, or fill the...
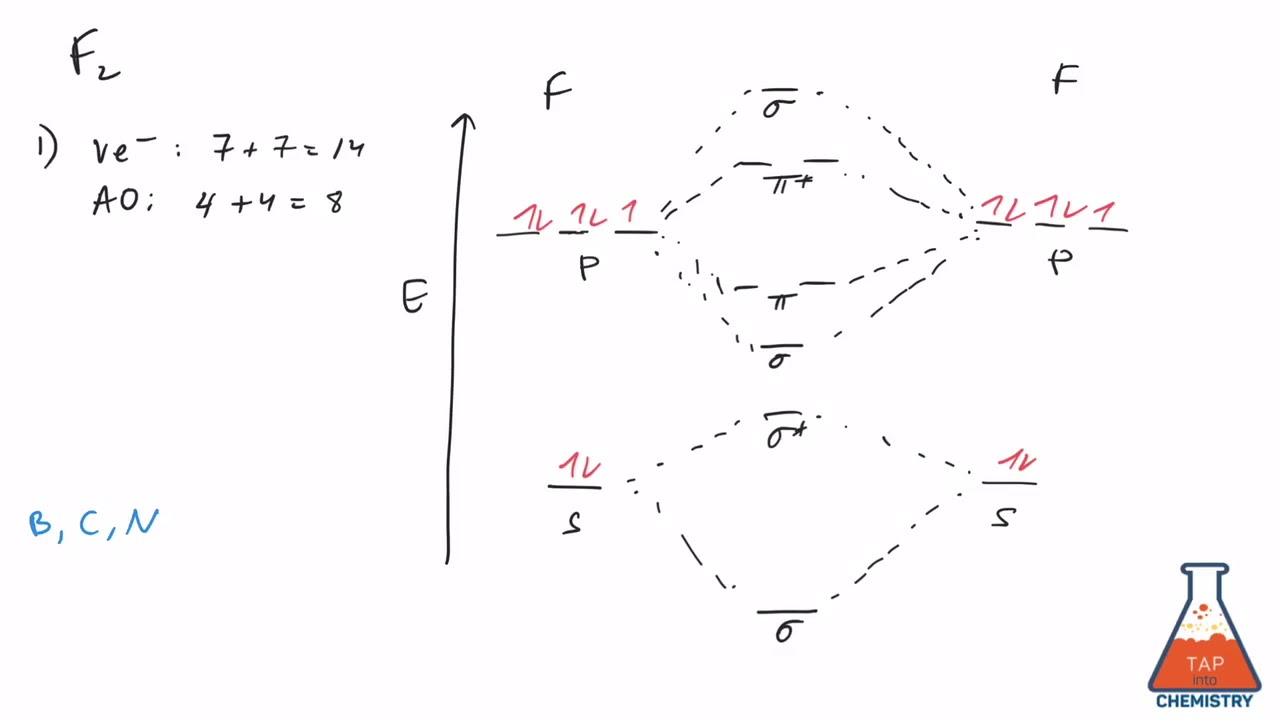
3. Construct the MO diagram for F2 (containing atomic orbitals, molecular orbitals, lines denoting which atomic orbitals contribute to which molecular orbitals, symbols for each molecular orbital, and placing electrons in the correct molecular orbitals)(10 points) a.
F2 MO diagram.pdf -. School Pace University. Course Title CHE 223. Uploaded By jmspigs1. Pages 1. This preview shows page 1 out of 1 page. View full document. End of preview. Want to read the entire page?
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...
The Best and Completed Full Edition of Diagram Database Website You Can Find in The Internet ... Gt 5000 Wiring Diagram Oldsmobile Cutlass Wiring Diagram Wiring Diagram Campbell Diagram To Dawn Control Wiring Diagram Usb Camera Wiring Diagram Plug Bone Diagram Chevy Bel Air Wiring Diagram Bladder Diagram Air Conditioning Parts Diagram Amp 120 Volt Plug Wiring Diagram Gear Motor Wiring Diagram Honda Odyssey Engine Diagram Jetta Ac Wiring Diagram Motor Capacitor Wiring Diagram Deluxe Telecaster S1 Wiring Diagram Honda S2000 Engine Wiring Diagram F150 Interior Fuse Panel Diagram Pt Cruiser Fus
As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond.
Jul 03, 2017 · For example, an ns/ns overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this: and an np/np overlap for O2 and F2 gives: So, the full MO diagram is: Thus, the valence electron configuration is: (σ2s)2(σ* 2s)2(σ2pz)2(π2px)2(π2py)2(π* 2px)2(π* 2py)2. Answer link.
Answer (1 of 3): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ...






















Comments
Post a Comment