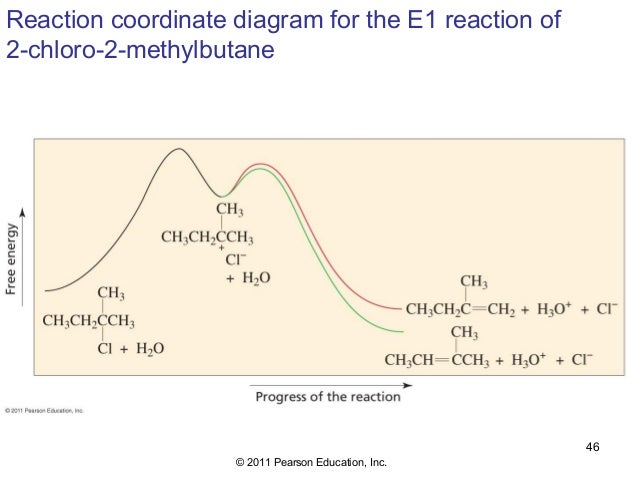
40 e1 reaction diagram
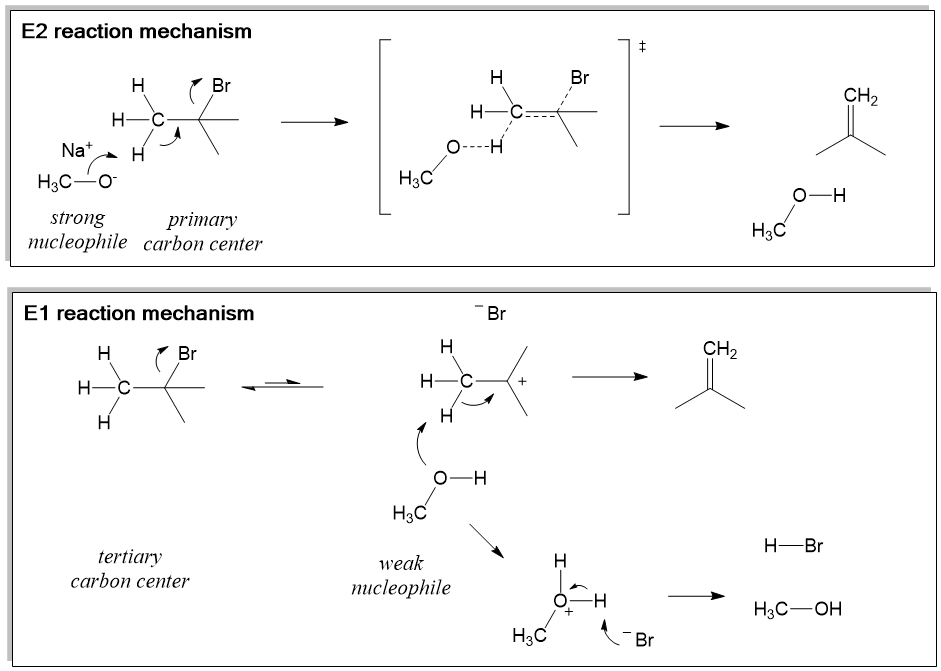
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate. Pyruvate dehydrogenase is usually encountered as a component, referred to as E1, of the pyruvate dehydrogenase complex (PDC). PDC consists … The bromide has already left so hopefully you see why this is called an E1 reaction. It's elimination. E for elimination and the rate-determining step only involves one of the reactants right here. It didn't involve in this case the weak base. Now that the bromide has left, let's think about whether this weak base, this ethanol, can actually do ...
Factors Affectin g the Rate of an E1 Reaction The rate of an E1 reaction increases as the number of R groups on the carbon with the leaving group increases. Increasing rate of E1 reaction RCH 2 XR 2CH XR 3C X 1° 2° 3° + + + I i bili f b i RCH 2 R 2CH R 3C 1° 2° 3° ncreas ng sta ty o car ocat ons The strength of the base usually determines ...

E1 reaction diagram
8.2 E1 Reaction. E1 Mechanism. Similar to substitutions, some elimination reactions show first-order kinetics. These reactions go through E1 mechanism, that is the multiple-step mechanism includes the carbocation intermediate. When t -butyl bromide reacts with ethanol, small amount of elimination products obtained via E1 mechanism. Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state ... 19/09/2012 · The E1 Reaction – Three Key Pieces of Evidence, and a Mechanism. Last time in this walkthrough on elimination reactions, we talked about two types of elimination reactions. In this post, we’re going to dig a little bit deeper on one type of elimination reaction, and based on what experiments tell us, come up with a hypothesis for how it works.
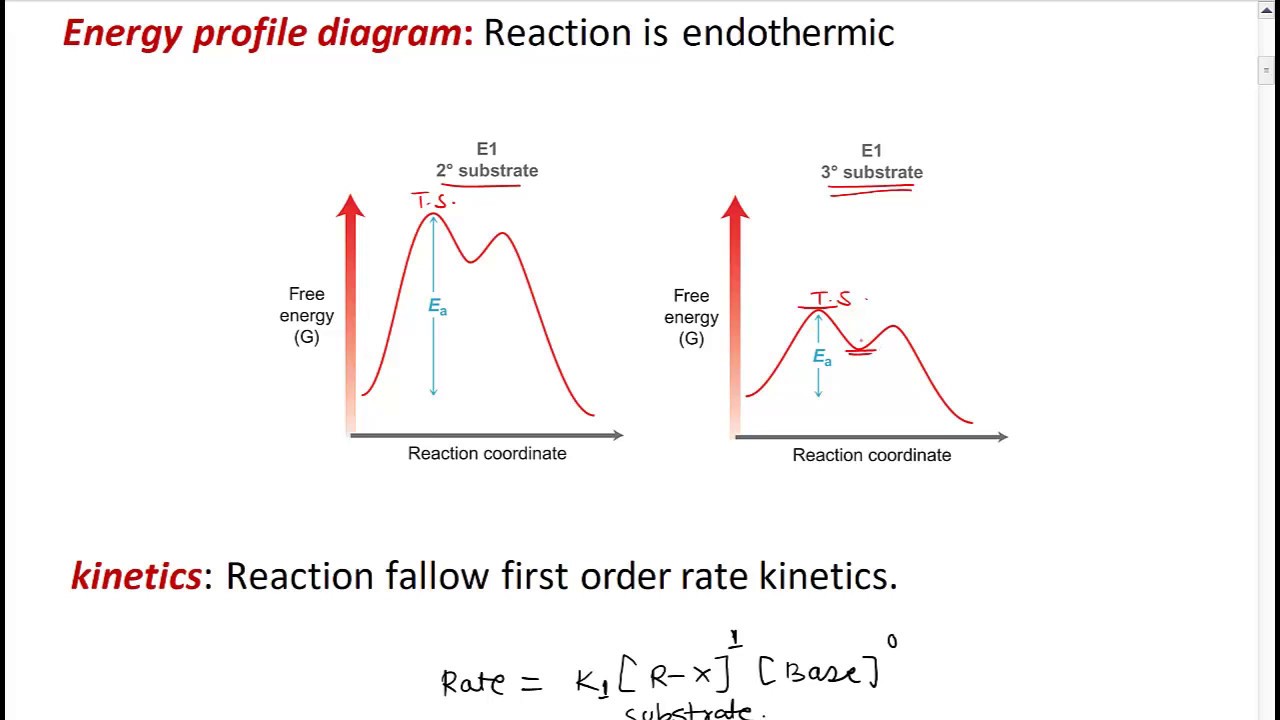
E1 reaction diagram. proton added to the leaving group to form water which then continues with the E1 reaction with water acting as the base/nucleophile. what is the overall process in the dehydration of an alcohol. no, strong base. ... what does the energy diagram for E1 look like. E1. what reaction does Zaitsev's rule only apply to. more substituted. In a multistep reaction, the rate-determining step does not necessarily correspond to the highest Gibbs energy on the reaction coordinate diagram. [5] [3] If there is a reaction intermediate whose energy is lower than the initial reactants, then the activation energy needed to pass through any subsequent transition state depends on the Gibbs ... E1 Elimination Reactions O •This E1 reaction diagram is similar to that of the S N1 reaction diagram. •E1 and S N1 reactions often compete with each other. 17. E1 Elimination Reactions O •Substrates that can form stable carbocation intermediates (secondary, tertiary, allylic, and benzylic) Energy Diagrams: Describing Chemical Reactions. Energy changes accompany chemical reactions. Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (E a), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as ...
Jul 13, 2012 · 1. Stereochemistry Of The SN1 Reaction: A Mixture of Retention and Inversion is Observed. If we start with an enantiomerically pure product, (that is, one enantiomer), these reactions tend to result in a mixture of products where the stereochemistry is the same as the starting material (retention) or opposite (inversion). Substitution Reactions (SN2 versus SN1) SN2. SN1. Elimination Reactions: E2 versus E1. Substrate: Alkene Stability Generic Reaction-Energy Diagrams. The central carbon is sp 2 -hybridized, whereat the p orbital is used for the partial Fig Transition state and energy diagram of an S N 2 reaction: Chloroform. In the E1 mechanism, the the first step is the loss of the leaving group, which leaves in a very slow step, resulting in the formation of a carbocation. The base then attacks a neighboring hydrogen, forcing the electrons from the hydrogen-carbon bond to make the double bond. 10/10/2012 · The E1 reaction is not very useful synthetically for olefin synthesis, because the ratio of elimination to substitution products is substantially lower than in the E2 reaction. For example, solvolysis of t-butyl bromide in dry ethanol only yields 19% isobutylene, whereas 93% yield of the alkene is obtained with 2M ethoxide. Mechanisms of elimination reactions. XIII. Effect of base, …
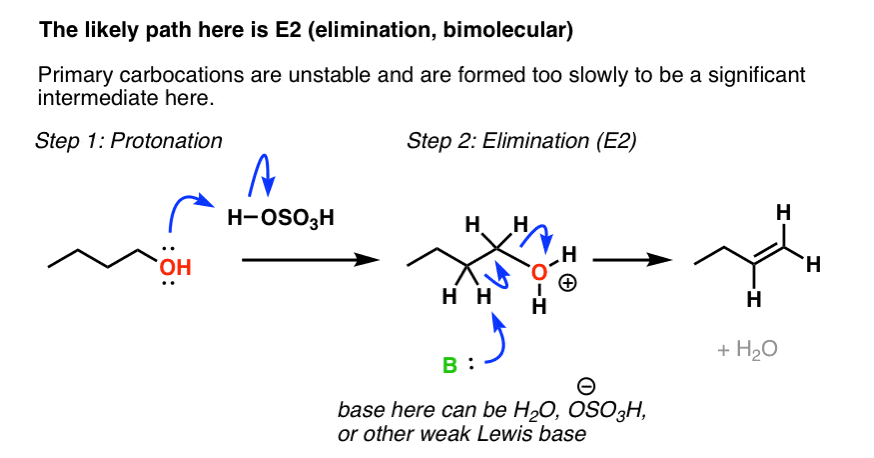
The E1 reaction proceeds via a two-step mechanism: the bond to the leaving group breaks first before the π bond is formed. The slow step is unimolecular, involving only the alkyl halide. 7. The dehydrohalogenation of (CH3)3CI with H2O to form (CH3)2C=CH2 can be used to illustrate the E1 mechanism. 8. Energy diagram for E1 reaction 9. 1. E1 Reaction In the E1 mechanism which is also known as unimolecular elimination, there are usually two steps involved - ionization and deprotonation. During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation. Jul 27, 2015 · The Alcohol Reaction Map Having now finished (at long last) covering the key reactions of alcohols, let’s stop to put everything in perspective. Over the past 18 articles (!) in this series on alcohols, we covered reactions such as: Substitution Reactions (SN2 versus SN1). Substrate: ... Elimination Reactions: E2 versus E1. Substrate: ... Elimination. Generic Reaction-Energy Diagrams.2 pages
e1 temperature, coarse pearlite forms at close to A e1 temperature due to low driving force or nucleation rate. At higher under coolings or lower temperature finer pearlite forms. At the nose of TTT diagram very fine pearlite forms Close to the eutectoid temperature, the undercooling is low so that the driving force for the transformation is ...
https://Leah4sci.com/elimination presents: E1 Reaction Coordinate Energy Diagram with step by step mechanism, transition states and intermediates📺Watch Next...
Pourbaix Diagram. 1. Battery equilibrium electrochemistry. Suppose an electrochemical reaction happening on the electrode/electrolyte interface: R O + ne. − (1) At equilibrium, the electrode potential is related to activities of the reactants by the Nernst equa tion: kT a. Oa. n. E = E. 0 + ln. e (2) ne a. R. where E. 0
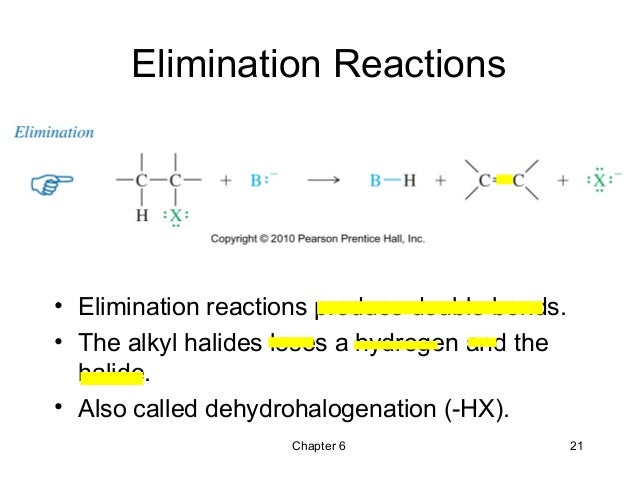
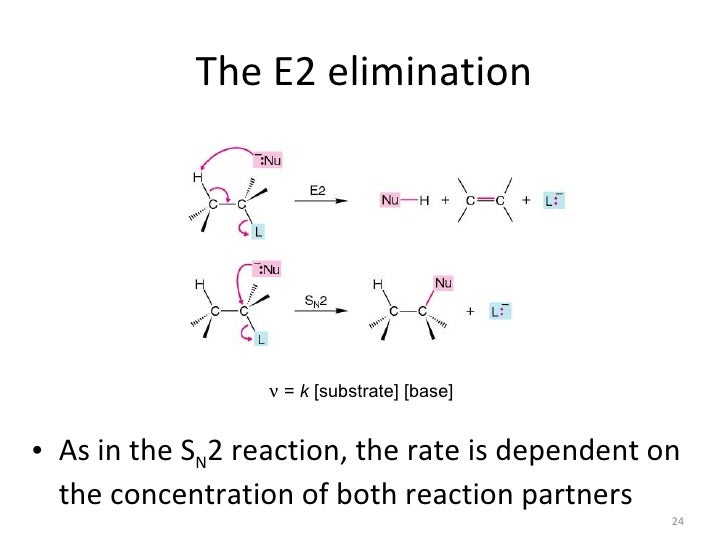
First of all, an elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism.. The one-step mechanism is known as the E2 reaction; The two-step mechanism is known as the E1 reaction. Note: The numbers do not have to do with the number of steps in the mechanism, but rather the kinetics of the reaction ...
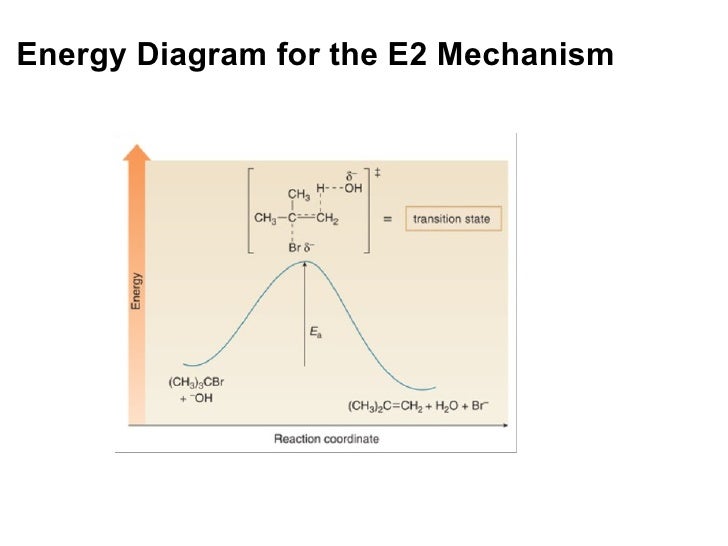
Figure 9.11 Reaction free-energy diagram for the S N1-E1 solvolysis reaction of (CH 3) 3CBr with ethanol.The rate-limiting step,ionization of the alkyl halide (red curve),has the transition state of highest standard free energy.The relative rates of the product-determining steps (blue curves) determine the relative amounts of substitution and
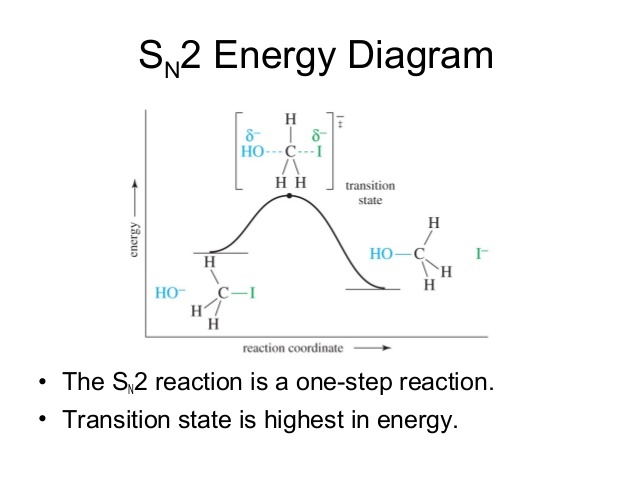
Energy Diagram For Sn2. It starts with the kinetics of SN2 reaction and covers the energy diagrams including questions on activation energy, enthalpy, the order of reaction and curved. SN2 Reaction follows second order rate kinetics. It forms a product via one transition state. Transition state is the state at which it posses.
Watch Complete videos @ www.LearningChemistryOnline.com
A) E1, SN1 B) E1, SN2 C) E2, SN1 D) E2, SN2. Predict the organic product (s) for the following reaction. Be sure to indicate stereochemistry when appropriate. If stereoisomers are produced draw one and label the relationship between the stereoisomers. (enantiomers, diastereomers, etc.). Draw the major organic product for the following reaction.
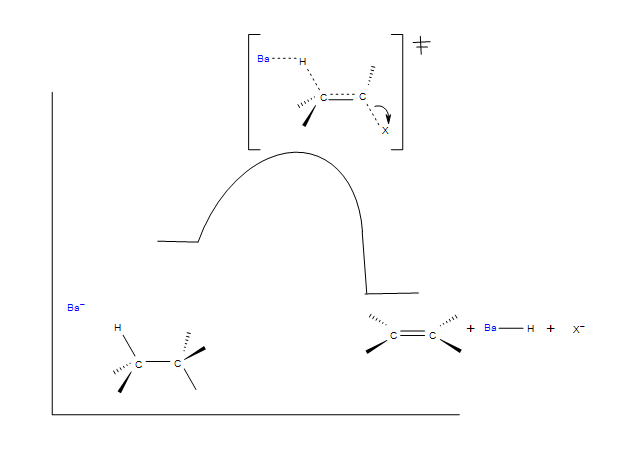
E2 mechanism is also a single-step concerted reaction, similar to S N 2, with multiple electron pair transfers happen at the same time.. Figure 8.1b E2 Reaction Mechanism. Base, OH –, uses its electron pair to attack a β-hydrogen on β-carbon, and starts to form a bond; at the same time, the β C-H sigma bond begins to move in to become the π bond of a double bond, and meanwhile Br begins ...
E1 reactions refers to elimination reactions of unimolecular organic reactions. Elimination reactions are a type of reactions in which the elimination of groups attached to the carbon atom takes place and a double bond formation occurs between two carbon atoms. ... 3 CBr].The potential energy diagrams for the reactions by taking reaction ...
Ubiquitin-activating enzymes, also known as E1 enzymes, catalyze the first step in the ubiquitination reaction, which (among other things) can target a protein for degradation via a proteasome.This covalent bond of ubiquitin or ubiquitin-like proteins to targeted proteins is a major mechanism for regulating protein function in eukaryotic organisms.
reaction if the reactant is not charged. Ex: H2O, CH3OH, etc. Racemization (with some inversion because of ion pairing) E1 3>2>1 Forms a carbocation Weak base favors E1 reaction by disfavoring E2 reaction Not effected but a low concentration of base favors E1 by disfavoring a E2 reaction Protic polar favors a E1 reaction if the reactant is not ...
A photosynthetic reaction center is a complex of several proteins, pigments and other co-factors that together execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems , give rise to electron ...
Potential Energy Diagram for E1 TS energy depends on carbocation rate-determining titi tt stability and leaving group quality. (Same as SN1.) H CH3 CH3 E transition state TS energy does not depend on the strength of the base. X H H a rate determining E - step reaction coordinate E1 and SN1 Frequently Occur Together (because they pass through a ...
The fast reaction of the carbocation with the nucleophile is the driving force of the S N 1 reaction since it pulls the equilibrium to the right according to the Le Châtelier’s principle. S N 1 – A Two-Step Mechanism. Let’s break down all the steps in the following S N 1 reaction looking at the energy diagram: Step [1] Breaking the C ...
Let’s break down the steps of the E1 reaction and characterize them on the energy diagram: Step 1: Loss of he leaving group. The energy diagram of the E1 mechanism demonstrates the loss of the leaving group as the slow step with the higher activation energy barrier: The dotted lines in the transition state indicate a partially broken C-Br bond. The Br being the more …
How the heck do you tell the difference between an E1, E2, SN1, SN2 reaction? Check out the chart below to start. The factors that will decide E1, E2, SN1, SN2: 1) Do you have a strong nucleophile? If you do, it will favor an SN2 reaction. If it is a mediocre nucleophile, it will favor an SN1 reaction. This is because of the two mechanisms.
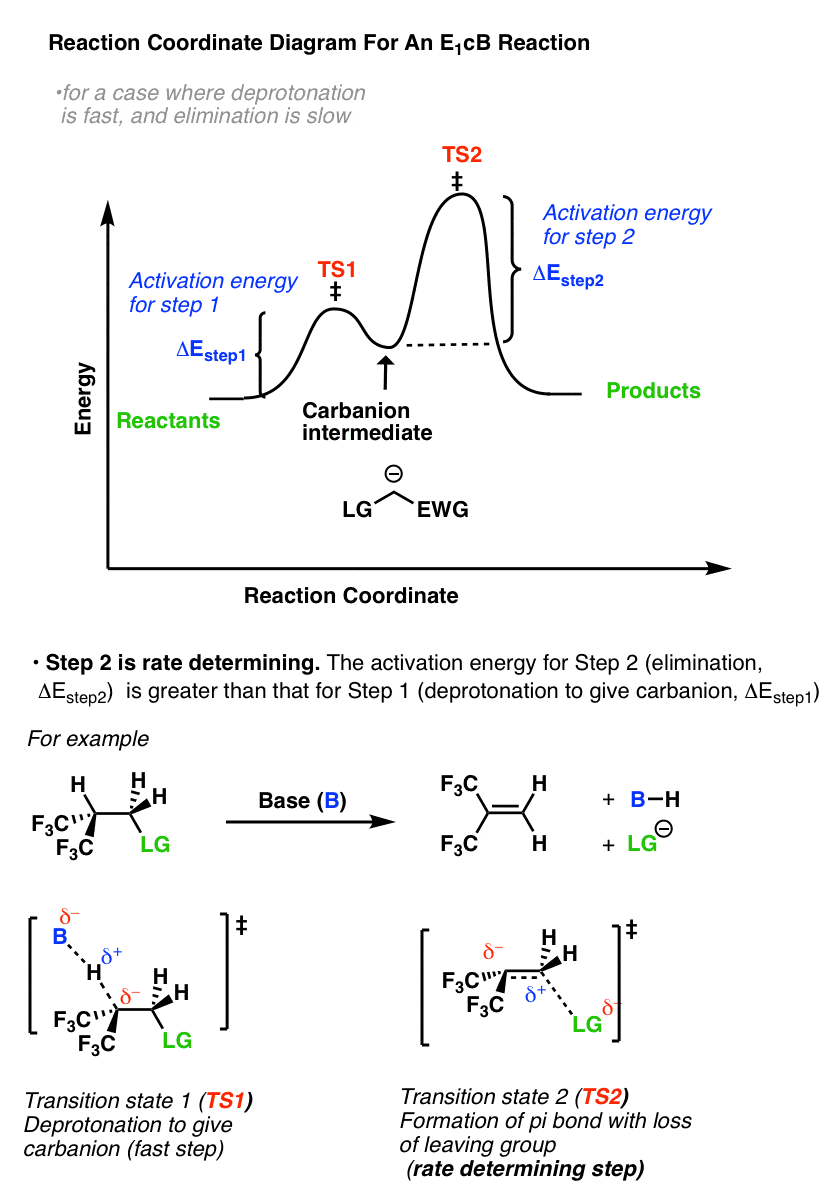
Energy profile diagram of E1 reaction: Orientation and reactivity of E1 reaction: References; Elimination reaction. An elimination reaction is a type of organic reaction in which a pair of atoms or group of atoms are removed from a organic molecule. Elimination reaction is the principal process by which saturated organic compounds (i.e ...
In E1 reactions these same substances would act as bases to capture a proton in the elimination step. Once formed, the carbocation can: a) Go on to form the Sn1 product. Remember that when the nucleophile is a neutral protic solvent, its conjugate base replaces the leaving group.
The key differences between the E2 and E1 mechanism are: 1) E2 is a concerted mechanism where all the bonds are broken and formed in a single step. The E1, on the other hand, is a stepwise mechanism. 2) E2 reactions are favored by strong bases such as the methoxide (MeO -), ethoxide (EtO - ), potassium tert-butoxide ( t BuOK), DBN, DBU, LDA ...
E1 reactions are two-step reactions, which means, an E1 reaction occurs through two steps named as ionization and deprotonation. In the ionization process, a carbocation is formed due to the removal of a substituent. In the second step (deprotonation), the carbocation is stabilized by removal of a hydrogen atom as a proton.
This page covers the mechanistically related reaction types, S N 1 and E1. Its purpose is to point out the similarities and differences between these two reaction types, as well as distinguish them from related S N 2 and E2 reactions. The reader is strongly encouraged to review the pages on S N 2 and E2 reactions along with this page. A brief summary of the four modes of reactivity follows the ...
E2 Reaction Energy Diagram (coming soon) Choosing SN1 SN2 E1 E2 Reaction Mechanism, Given Reactant and Product; Video 1 – Introduction To Substitution And Elimination Reaction Analysis. Many students read ‘introduction' and translate ‘skip this video' I'm here to tell you NOT TO. The introductory video is under 2 minutes long, and my goal ...
E1 Reactions Unimolecular elimination (E1) is a reaction in which the removal of an HX substituent results in the formation of a double bond. It is similar to a unimolecular nucleophilic substitution reaction (SN1) in particular because the rate determining step involves heterolysis (losing the leaving group) to form a carbocation intermediate.
the slow step of the reaction) and a weak electron pair donor in SN1/E1 reactions (that's why they don't participate in the slow step of the reaction). This leads to differences in reaction mechanisms, which show up in the kinetics of the rate law expression (bimolecular = 2 and unimolecular = 1) and the possible reaction products obtained.
May 30, 2020 — The unimolecular E1 mechanism is a first order elimination reaction in ... draw and interpret Reaction Energy Diagrams for E1 reactions.
The E1 mechanism is nearly identical to the S N 1 mechanism, differing only in the course of reaction taken by the carbocation intermediate. As shown by the following equations, a carbocation bearing beta-hydrogens may function either as a Lewis acid (electrophile), as it does in the S N 1 reaction, or a Brønsted acid, as in the E1 reaction.
19/09/2012 · The E1 Reaction – Three Key Pieces of Evidence, and a Mechanism. Last time in this walkthrough on elimination reactions, we talked about two types of elimination reactions. In this post, we’re going to dig a little bit deeper on one type of elimination reaction, and based on what experiments tell us, come up with a hypothesis for how it works.
Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state ...
8.2 E1 Reaction. E1 Mechanism. Similar to substitutions, some elimination reactions show first-order kinetics. These reactions go through E1 mechanism, that is the multiple-step mechanism includes the carbocation intermediate. When t -butyl bromide reacts with ethanol, small amount of elimination products obtained via E1 mechanism.

![E1 reaction - [PPTX Powerpoint]](https://reader016.documents.pub/reader016/slide/20190517/55507065b4c9052d158b4a66/document-17.png?t=1613946530)






















Comments
Post a Comment