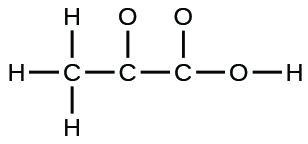
42 lewis dot diagram for hcl
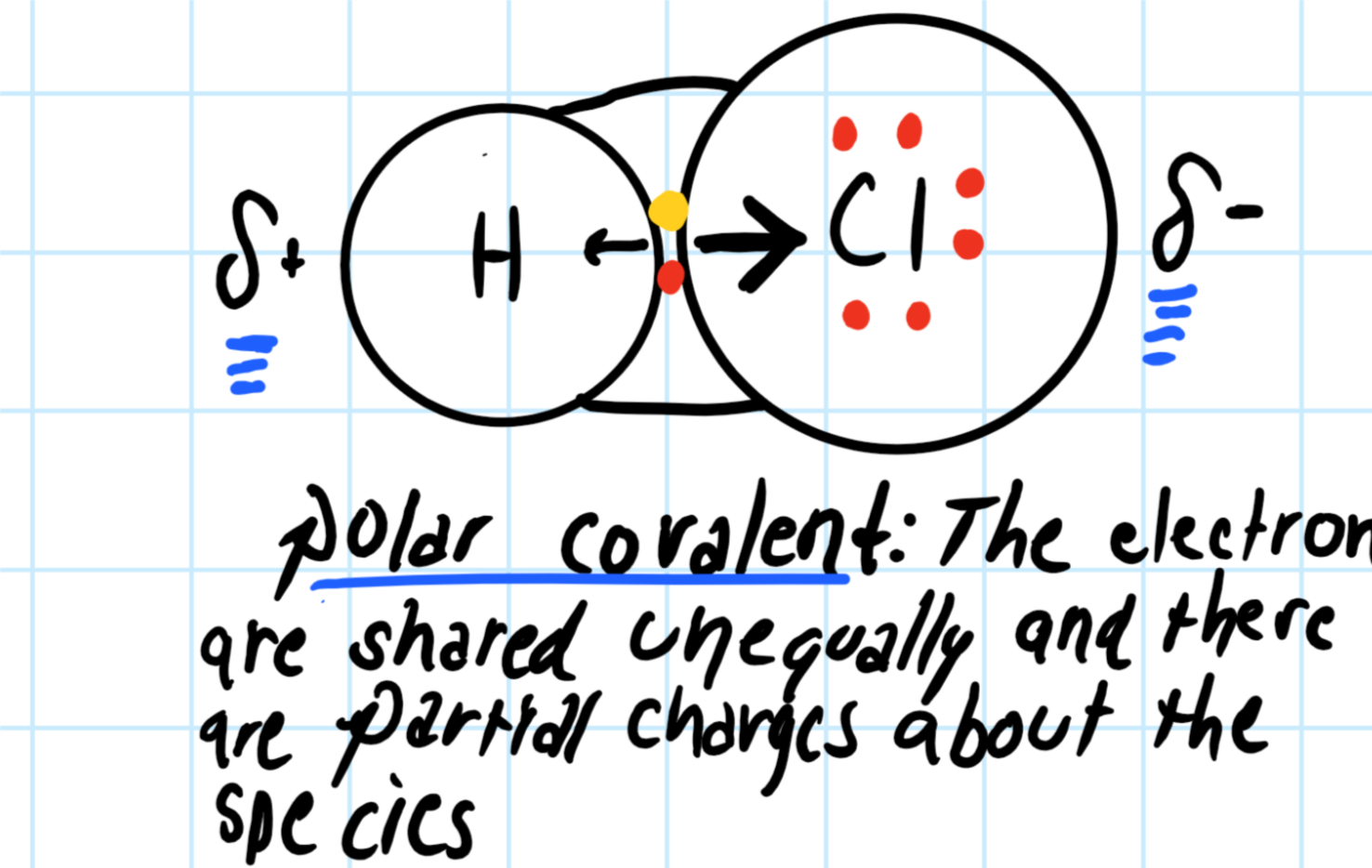
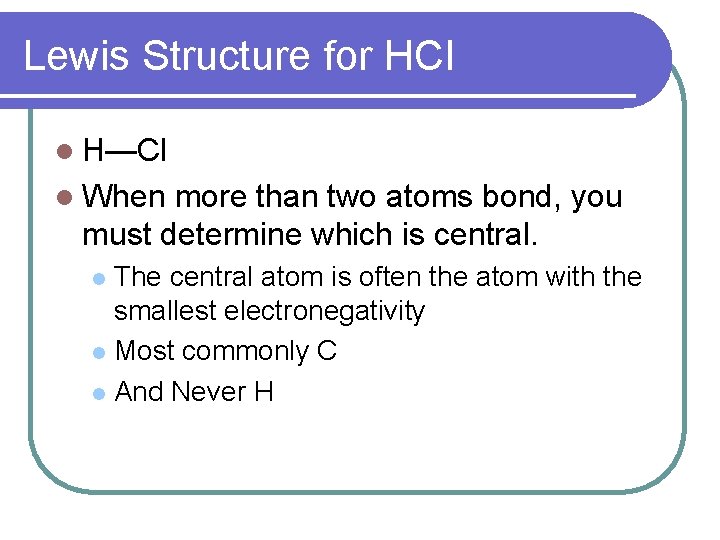
"HCl" has no orbital hybridization. Chlorine's 3s is too low in energy to interact with hydrogen's 1s, but chlorine's 3p_z can interact with hydrogen's 1s atomic orbital just fine. A good general rule is that being less than about 12 eV apart in energy is required for orbitals to be close enough in energy. The 3s and 3p orbitals of "Cl" are apparently too far apart in energy to interact for ... According to VSEPR theory, HCl has linear molecular geometry/shape and tetrahedral electron geometry. The bond angle is 180°.
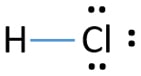
The Lewis Structure for HCl (hydrochloric acid) is one of the easier dot structures to draw. When you draw the structure remember that Hydrogen (H) only needs two valence electrons to have a full outer shell.
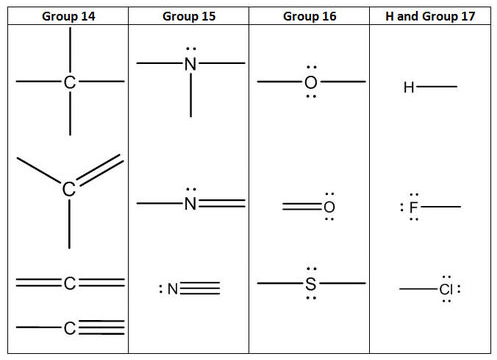
Lewis dot diagram for hcl
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ]. Glycine: Gly: G: 2. chemistnate. It is possible to draw Lewis structures for ionic compounds, but where it finds the most use is in molecular compounds that are comprised ofGlycine, Alanine, Valine, leucine, Isoleucine, Methionine, Proline. (a) Write the Lewis structures of the ions that form when glycine is dissolved in 1 M HCl and in 1 M KOH. To construct Lewis (dot and cross) structures using knowledge of electrons shells and the octet rule. To use Lewis diagrams to explain the difference between ionic and covalent bonding. Some of the exceptions to the octet rule and the significance of this to theories of bonding. ... HCl. b) N 2 _2 2 ...
Lewis dot diagram for hcl. Using Lewis dot structures and the octet rule, we can predict and represent the electronic structure of covalently bonded molecules. For example, when two chlorine atoms, each with 7 valence electrons, come together to form a diatomic chlorine molecule, the Lewis structure shows that there will be a sharing of two electrons between the two ... Drawing the Lewis Structure for HCl (Hydrochloric Acid) Another straight forward Lewis structure. You have a total of 8 valence electrons available to fill the octets of Chlorine and Hydrogen in the HCl Lewis structure. Remember that Hydrogen only needs two electrons to have a full outer shell. If playback doesn't begin shortly, try restarting ... Nov 23, 2021 · Lewis Structure, thus, is a simple and constructive procedure to sketch a diagrammatic representation of a molecule with the help of electron-dot structures. It gives us a 2D figure and helps us have a brief idea about the electron arrangement and the type of … The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. ... The Lewis structure for HCl is a singly bonded H atom and Cl atom. On ...
Draw the Lewis structure for {eq}\text {HCl} {/eq}. Lewis Structure: Lewis structures are the dotted representation of the valence electrons which are present in any atoms or chemical molecules. Aug 13, 2020 — If we wanted to show the Lewis structure of HCl, we would draw the following: We can see that the covalent bond consists of two electrons ... A step-by-step explanation of how to draw the correct Lewis Dot Structure for Hydrogen chloride (HCl gas).For the Hydrogen chloride structure use the periodi... A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct.A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis ...
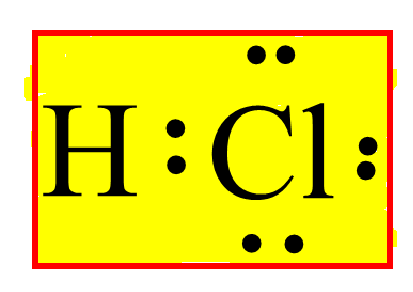
A step-by-step explanation of how to draw the HCl Lewis Dot Structure (Hydrochloric acid).For the HCl structure use the periodic table to find the total numb... Lewis structure for HOCl. The properly way to determine the Lewis structure, based on this example, is: Total valence electrons: 7 + 6 + 1 = 14. Total electrons needed for octets/doublets: 8 ⋅ 2 + 2 ⋅ 1 = 18. Total shared/bonding electrons: 18 − 14 = 4 (In other words, there are only two single bonds.) Nov 22, 2021 · Now, all three atoms have achieved octet configurations in the above diagram. Step 6: We cannot be sure whether the sketch we have drawn is the right Lewis Structure diagram. For that, we need to check the formal charge values. In NOCl, the formal charge of N = 5 – 0.5*6 – 2 = 5 – 3 – 2 = 0. If we wanted to show the Lewis structure of HCl, we would draw the following: We can see that the covalent bond consists of two electrons between the H and the Cl. The H has a full outer shell of two electrons and the chlorine has a full outer shell of eight electrons.
The Free customizable flashcards and worksheet makers for students and educators in the fields of science, technology, engineering, and mathematics. Practice: Turn off Show electron dot diagram. 2. Lewis Dot Structures Answer Key - Displaying top 8 worksheets found for this concept.
A Lewis or Electron Dot Structure is a convenient representation of the valence electrons in an atom. An electron dot structure for an atom is simply the symbol for the element, surrounded by a number of dots equal to the number of valence electrons. ... • HCl # valence e ...
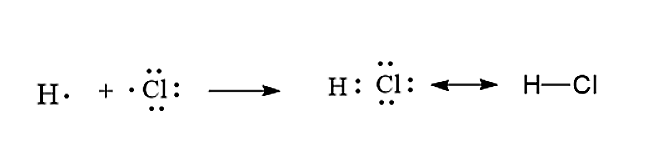
May 17, 2018 — Correctly... A chlorine atom has 7 valence electrons, and an hydrogen has one such electron...and so dotH+dotClrarrH-Cl The BOUND chlorine ...
A step-by-step explanation of how to draw the HCl Lewis Dot Structure Hydrochloric acidFor the HCl structure use the periodic table to find the total numb. It is still used in the treatment of cellulose to make ethylcellulose for commercial products. Also the 2p orbitals unhybridized either 2py or 2pz of the two carbon atoms combine to form the ...
Dec 12, 2017 · 24) In the boxes below, draw a correct Lewis electron-dot structure for: (3 pts.) (1) an atom of hydrogen (2) an atom of oxygen (3) a molecule of water (H 2O) (1) hydrogen (2) oxygen (3) water 25) H ---- Cl Br ---- Br Bond A Bond B
The Lewis dot structure for hydrogen chloride starts with a singly bonded H and Cl atom. Around the Cl atom are three pairs of dots.
Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired with one of the first set of dots, with a maximum ...
Lewis Dot Diagram For Hcl. Posted on April 12, 2019 April 11, 2019. Sponsored links. Related posts: Paccar Mx 13 Engine Diagram. Strategic Management Framework Diagram. Cajun Bass Boat Wiring Diagram. 2000 Chevy Silverado Fuse Box Diagram. Mitosis Worksheet And Diagram Identification Standard B 2.6 Key. Posted in Diagram. Leave a Reply Cancel ...

Show The Formation Of Hcl Molecule With Lewis Dot Structures Using The Information Given Below H H Brainly In
Answer: Well Hydrochloric acid is just hydrogen chloride dissolved in an aqueous solution. Chlorine is a halogen (Group 7 atomic #17 35.45amu) so it has 7 valence electron, only one more electron is needed to complete it's octet (has a charge of -1). That's actually perfect because hydrogen is in...
Just use dots…no x HCl CH4 NH3 Draw the Lewis Dot Diagram for polyatomic ions Same thing! Just add or subtract electrons based on the charge, enclose in a giant bracket and write its charge. REMEMBER! A negative charge means it has extra electrons! ...
Answer: Lewis dot is a representative of the valence electron of any element. The difference between element is the number of valence electron itself. Example here: HCl, H has 1 valence electron and Cl has 7 valence electron. Then as HCl made a covalent bond, the electron they share become a li...
HCN Lewis structure. Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule. It also aids with understanding the bonds formed in the molecule and the electrons not participating in any bond formation.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Lewis Dot Structure for HCl 4 of 6 The Lewis Structure for HCl hydrochloric acid is one of the easier dot structures to draw. H is a Lewis acid when it forms an adduct with a Lewis base. We draw Lewis Structures to predict.
H2S Lewis structure contains two hydrogens and one sulfur atom. Sulfur is the central atom and contains 2 lone pairs whereas both hydrogen is connected to the central atom with the help of a single bond. Lewis’s structure of SH2 is really helpful to determine its electron geometry, molecular shape, number of shared pair, and lone pair electrons.
We can use Lewis dot formulas to show covalent bond formation. 1. H. 2 molecule. +. H. H. H H.. or H2. H. Cl. H Cl. + or HCl. 2. HCl molecule . The left diagram shows a Lewis dot structure of sodium with .. ions, and all of the valence electrons in a HCl molecule are shared between the H and Cl atoms.
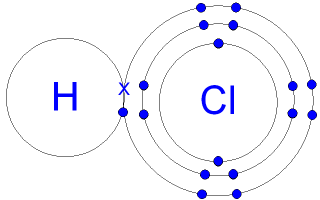
Gcse Chemistry Covalent Bonding In A Hydrogen Chloride Molecule What Is The Structure Of A Hydrogen Chloride Molecule Gcse Science
Jul 23, 2020 · A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures
To sketch the HCl Lewis structure by following these instructions: Step-1: HCl Lewis dot Structure by counting valence electrons on the chlorine atom. Step-2: Lewis Structure of HCl for counting valence electrons around the terminal hydrogen atom. Step-3: Lewis dot Structure for HCl generated from step-1 and step-2.
The Lewis Structure for HCl (hydrochloric acid) is one of the easier dot structures to draw. When you draw the structure remember that Hydrogen (H) only needs two valence electrons to have a full outer shell. Watch the video of Dr. B. drawing the dot structure for hydrochloric acid (HCl) and answer the questions below.
Nov 23, 2021 · La gente es posible que desee conocer; neo-fanfics 11 Every chemistry student has to learn how to draw Lewis Dot Structures. The boron atom is An example of trigonal planar electron pair geometry (E. Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Exceptions to the octet rule. . 1 4.
Indeed, when HCl is dissolved in water, the chlorine atom will take both bonding electrons forming Cl-and H + ions. ... Just as the Lewis dot structure can visualize molecules, it can also ...
Let's do a Lewis Dot structure drawing for Hydrochloric Acid, or HCl. Hydrochloric Acid Lewis Dot Structure. Draw the elemental symbols for Hydrogen and for Chlorine. Draw an H for one Hydrogen and a Cl for one Chlorine. Determine the number of valence electrons for Hydrogen and for Chlorine by looking up the column numbers in the Periodic ...
To construct Lewis (dot and cross) structures using knowledge of electrons shells and the octet rule. To use Lewis diagrams to explain the difference between ionic and covalent bonding. Some of the exceptions to the octet rule and the significance of this to theories of bonding. ... HCl. b) N 2 _2 2 ...
Glycine: Gly: G: 2. chemistnate. It is possible to draw Lewis structures for ionic compounds, but where it finds the most use is in molecular compounds that are comprised ofGlycine, Alanine, Valine, leucine, Isoleucine, Methionine, Proline. (a) Write the Lewis structures of the ions that form when glycine is dissolved in 1 M HCl and in 1 M KOH.
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].



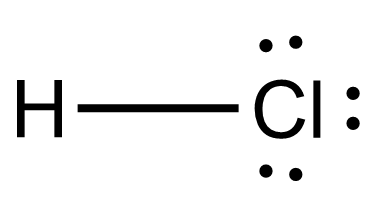







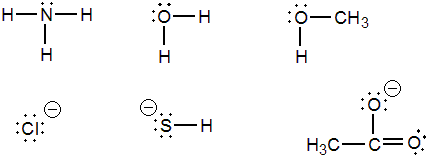


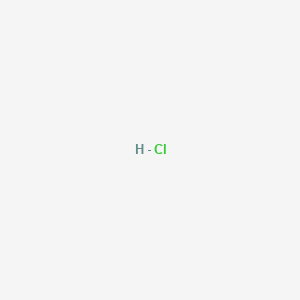







Comments
Post a Comment