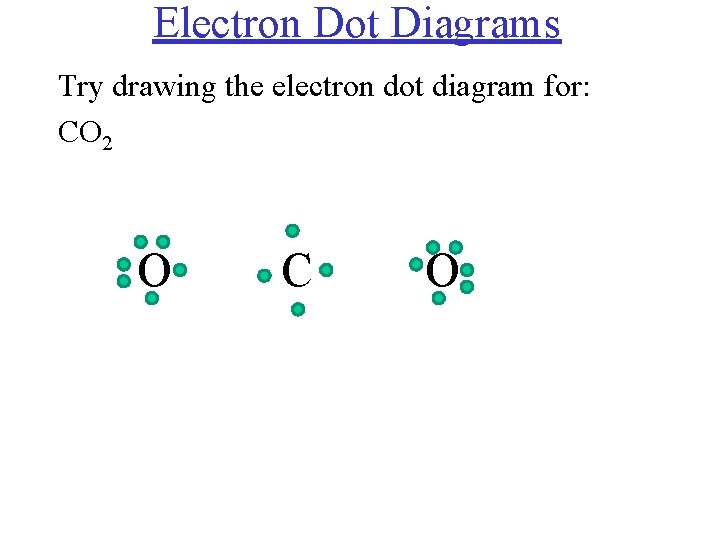
38 electron dot diagram for co2
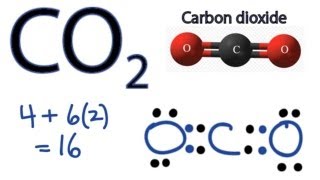
Answer: CO2 Lewis structure (carbon dioxide electron dot structure) is that type of diagram where we show the total 16 valence electrons of CO2 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ]. CO2 Lewis Structure. The lewis structure of CO2 can be with some simple steps, but before that, it is important to understand lewis structure properly. So lewis structure generally gives us an idea about the nature of bonding and octet fulfillment of the atoms. According to the octet rule, an atom attains stability by fulfilling its octet.
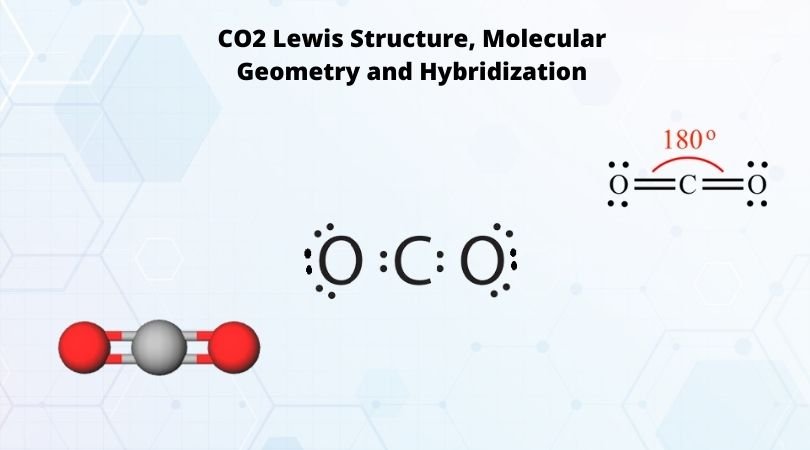
Carbon Dioxide is a Linear molecule, with AX2 geometry, a linear shape, and a 180 degree bond angle.Check me out: http://www.chemistnate.com
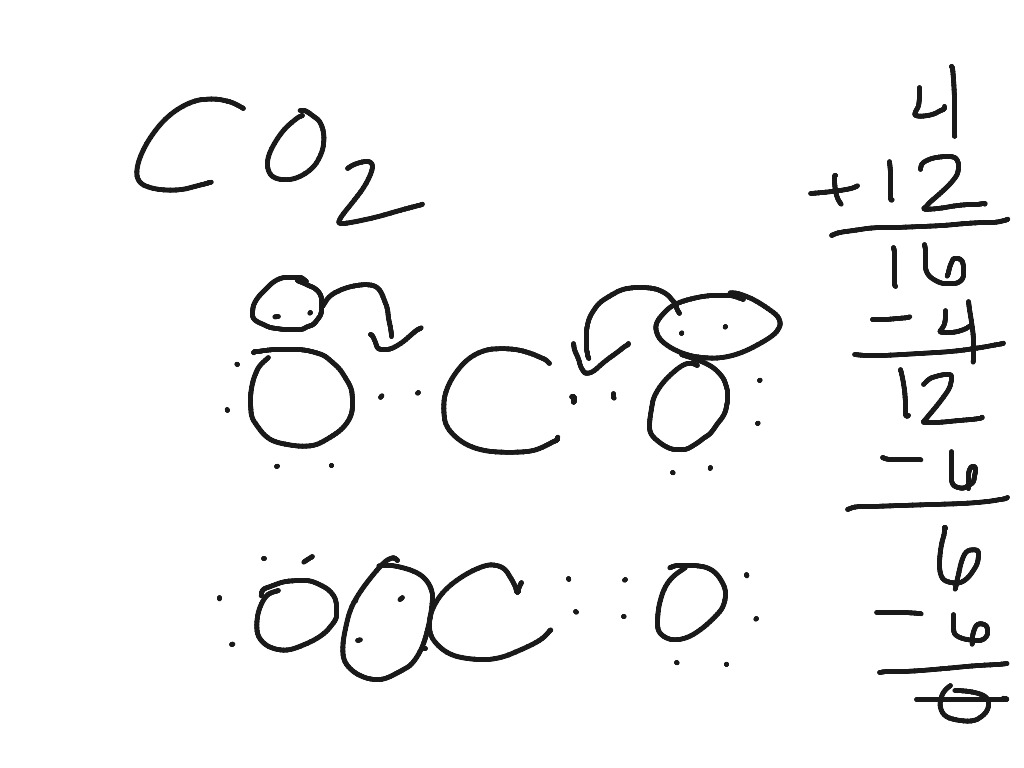
Electron dot diagram for co2
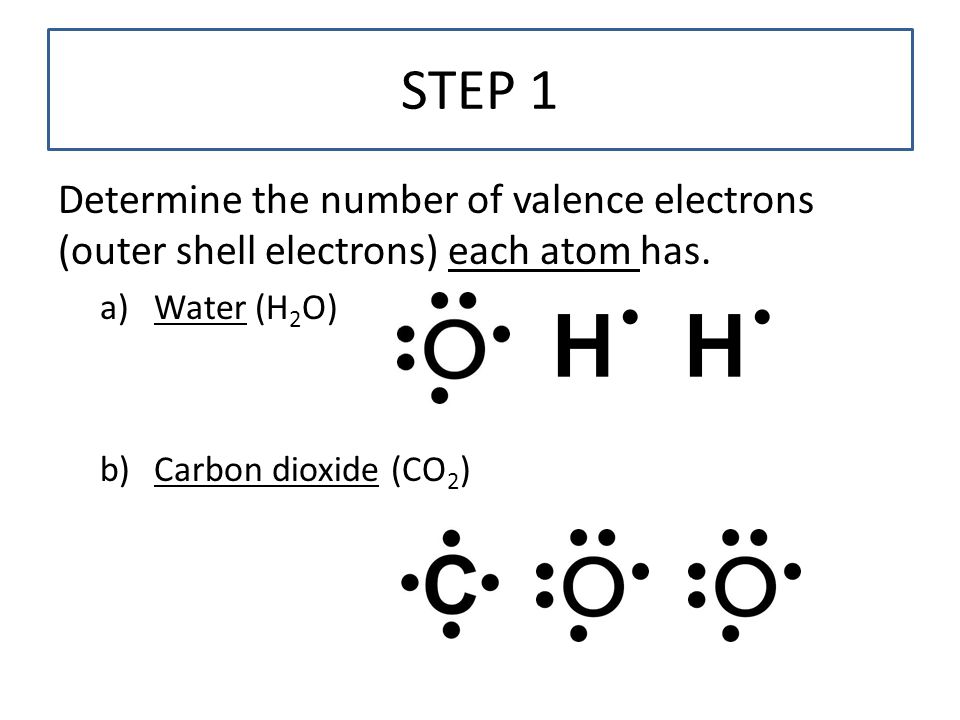
To sketch the CO2 Lewis structure by following these instructions: Step-1: CO2 Lewis dot Structure by counting valence electrons on the carbon atom. Step-2: Lewis Structure of CO2 for counting valence electrons around the terminal oxygen atoms. Step-3: Lewis dot Structure for CO2 generated from step-1 and step-2. Lewis dot structure of OF2 plays an important role to determine the geometry of it because it helps to find how many bond pair and lone pair electrons OF2 molecule contains. Follow three steps to find OF2 molecular shape and its electron geometry 1. Find the Number of lone pairs present on the central atom of the OF2 lewis structure This structure helps in knowing the arrangement of electrons in the molecules and the shape of the molecule. To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule.
Electron dot diagram for co2. Carbon dioxide (CO2) lewis structure has two double bonds around carbon atom. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO2 is linear. Steps of drawing the lewis structure of CO2 are explained. CO2 Lewis Structure and Molecular Geometry. Carbon dioxide (CO2) is a colourless, odourless, incombustible gas resulting from the oxidation of carbon. Its Lewis structure comprises two different atoms: carbon, and oxygen. It is a nonpolar molecule with bond angles of 180 degrees. CO2 is used as the refrigerant in fire extinguishers and it is a ... Draw the electron dot diagram for the CH_3^- molecule and determine its VSEPR shape. View Answer. Give the shapes of CH_3, O_3, SnCl_3, BrF_3, I_3. View Answer. What are the primary tools used to ... Even‑electron species may be either diamagnetic or paramagnetic, so you will need to fill in the diagram to know for sure. F2 has no unpaired electrons and is diamagnetic. F+2 has …
What is the electron dot diagram for carbon? What is a Lewis dot diagram? What is the Lewis structure for #CN_2H_2#? See all questions in Lewis Dot Diagram Impact of this question. 15978 views around the world You can reuse this answer Creative Commons License iOS ... CO2 (Carbon Dioxide) Lewis Dot Structure. ... Though a Lewis structure may seem intimidating at first glance, it is actually fairly easy to understand a Lewis structure and to draw one yourself, if you break down the creation of the Lewis structure into simple steps. ADVERTISEMENT. Based on formal charges, which of the following is the best Lewis electron-dot diagram for H3NO? Diagram A. Which of the Lewis diagrams shown above is the more likely structure of CO2, and why? Diagram 1, because all the atoms have a formal charge of 0 . Which of the following Lewis diagrams represents a molecule that is polar? Diagram C. Which of the following correctly compares the … Draw the electron - dot structure of CO2 ? >. 11th. > Chemistry. > Chemical Bonding and Molecular Structure. > Basics of Chemical Bonding.
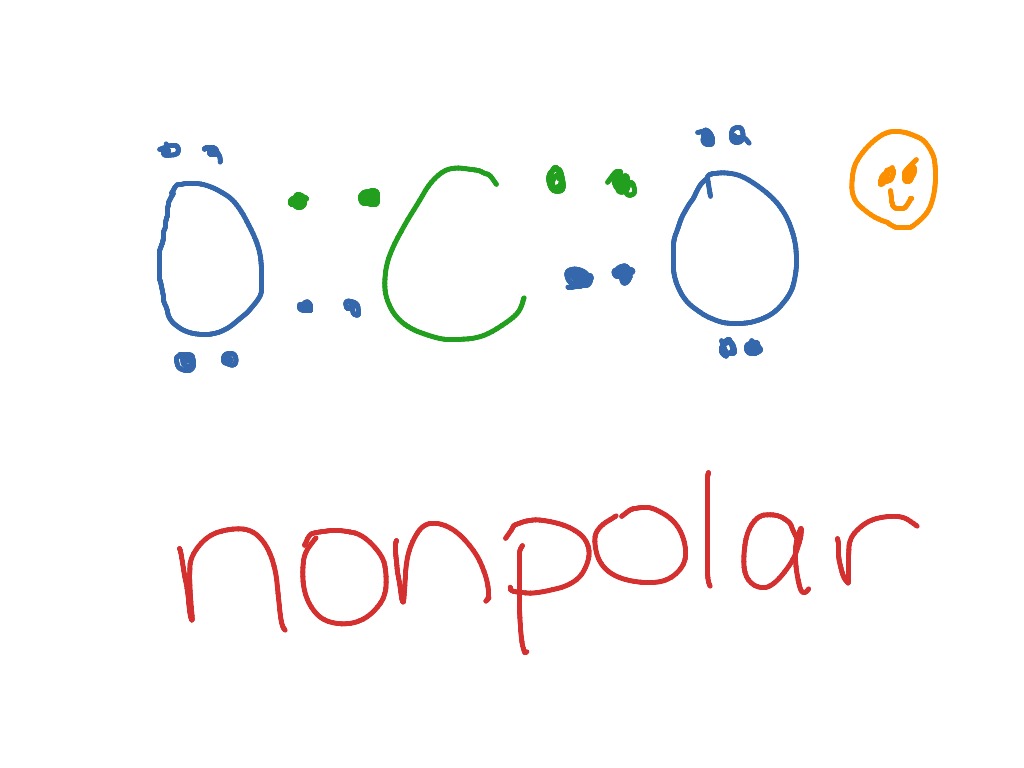
The net dipole moment of CO2 is zero. The electron and molecular geometry of CO2 are linear. The bond angle of CO2 is 180º and the hybridization of it is Sp. The total valence electron available for the CO2 lewis structure is 16. The overall formal charge in the CO2 lewis dot structure is zero. Carbon dioxide is a non-polar molecule. Co2 lewis dot structure the lewis structure of co2 looks something like that carbon c is at the center from carbon draw a two lines to each of the two oxygen o atoms surrounding it each of the two lines look like a long equal sign each line symbolizes one bond whats the lewis dot structure for carbon dioxide co2 o c o this is the lewis. Solubility for the CO2 as represented by the lewis structure is measured based on the composition in terms of the mole fraction. The mole fraction refers to the ratio between the number of moles of CO2 in solution and the number of moles in total. Even at the highest pressure ratios the mole fraction only reaches 8.00*10^-3 in terms of mole ratio. Electron Dot Diagram For Methane. Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only has one valence electron and can only share one. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown.
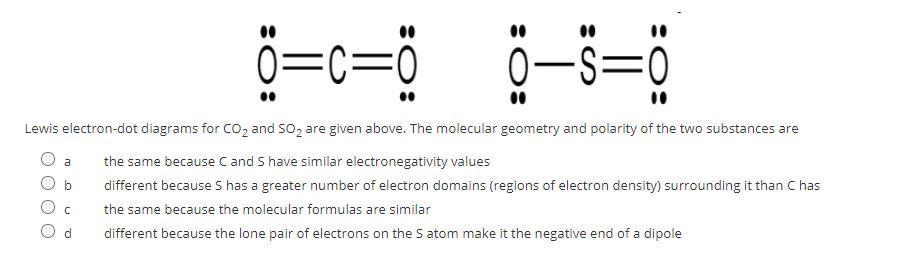
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds.

Draw The Lewis Dot Structure For Co2 Determine The Electron Geometry And Molecular Shape Of This Molecule Is This Molecule Polar Or Nonpolar Study Com
I quickly take you through how to draw the Lewis Structure of CO2 (Carbon DiOxide). I also go over hybridization, shape and bond angles.
In carbon dioxide molecule, the two oxygen atoms are bonded on either side with carbon atom by double bonds. Thus there are 2 double bonds in CO 2. . . Carbon share its two electrons In the formation of a double bond with one oxygen atom and another two electrons with another oxygen atom. In this process, both the oxygen atoms and the carbon ...
01.06.2020 · Metal–organic frameworks (MOFs) have attracted much attention in photo- and electrocatalytic CO 2 reduction into value-added chemicals. In this review, we specially focus on the active sites of MOF-based materials to achieve visible-light absorption and efficient charge separation for photocatalytic CO 2 reduction, and conductivity for electrocatalytic CO 2 reduction, respectively.
Carbon (C) is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structure. The Lewis structure for CO2 has a total of 16 valence electrons. In order to complete the octets for all of the atoms in the structure you will need to form two double bonds.
The schematic diagram symbol for a proximity switch with mechanical contacts is the same as for a mechanical limit switch, except the switch symbol is enclosed by a diamond shape, indicating a powered (active) device: Many proximity switches, though, do not provide “dry contact” outputs. Instead, their output elements are transistors configured either to source current or sink current. The ...
Answer (1 of 2): In CO2 Lewis structure,there are four lone pairs.Each oxygen atom has two lone pairs around it.The carbon atom doesn't have any lone pair. In CO2 Lewis structure,the carbon atom follows the octet rule and two oxygen atoms also follow the octet rule. In CO2 Lewis structure,we g...
Which Lewis Electron-dot Diagram is Correct for Co2. co2 lewis dot structure the lewis structure of co2 looks something like that carbon c is at the center from carbon draw a two lines to each of the two oxygen o atoms surrounding it each of the two lines look like a long equal == sign each line symbolizes one bond whats the lewis dot structure for carbon dioxide co2 o c o this is the lewis ...
Let us consider the case of the Lewis electron dot structures of carbon monoxide CO. Carbon monoxide is an odorless, colorless, non-irritant gas. It is the most common cause of fatal poisoning in Britain today. It causes the accidental deaths of up to 50 persons each year in the U.K. alone and a much larger number of non-fatal poisonings.
Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen.
CO2 - C Mean Corbon And Corbon Have our Electron And O Means Oxygen. And Oxygen Have 6 Valence Electron And in Subscript oxygen mutiply with 2 So 6*2 = 12 And Add 4 Of Corbon. 4+6*2= 16. So The Co2 Lewis Structure is :..O=C=..O: . You Can Understand It With Image That Given Below. The Post. Draw Lewis Structure For Ccl4.
Draw the Lewis structure of carbonate ion (CO2- 3). Question: Draw the Lewis structure of carbonate ion (CO2- 3). This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Best Answer. This is the best answer based on feedback and ratings.
CO2 can be used to flood the surgical field during cardiac surgery. Because of its density, carbon dioxide displaces the air surrounding the open heart so that any gas bubbles trapped in the heart are carbon dioxide rather than insoluble nitrogen.Similarly, CO2 is used to de-bubble cardiopulmonary bypass and extracorporeal membrane oxygenation (ECMO) circuits.
CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.".
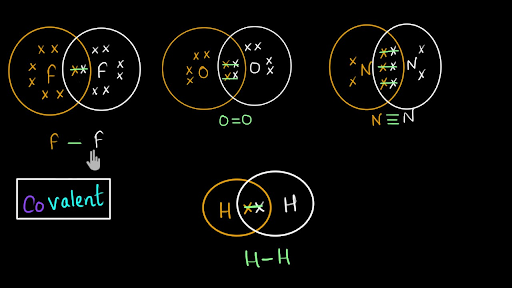
Cl2 Br2 H2 O2 N2 HCl DOUBLE bond atoms that share two e- pairs (4 e-) O O TRIPLE bond atoms that share three e- pairs (6 e-) N N Draw Lewis Dot Structures You may represent valence electrons from different atoms with the following symbols x, , CO2 NH3 Draw the Lewis Dot Diagram for polyatomic ions Count all valence e- needed for covalent bonding Add or subtract other electrons based on the ...
Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a shorthand notation to show the number of valence electrons in an atom. More complicated versions can be used to show the bond between different atoms in a molecule. The molecular formula for calcium chloride is CaCl2.
Lewis Structure for CO 3 2-| Carbonate ion. Lewis structure of carbonate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of CO 3 2-.After finishing the lewis structure of CO 3 2-, there should be a -2 charge and it should be stabile structure.
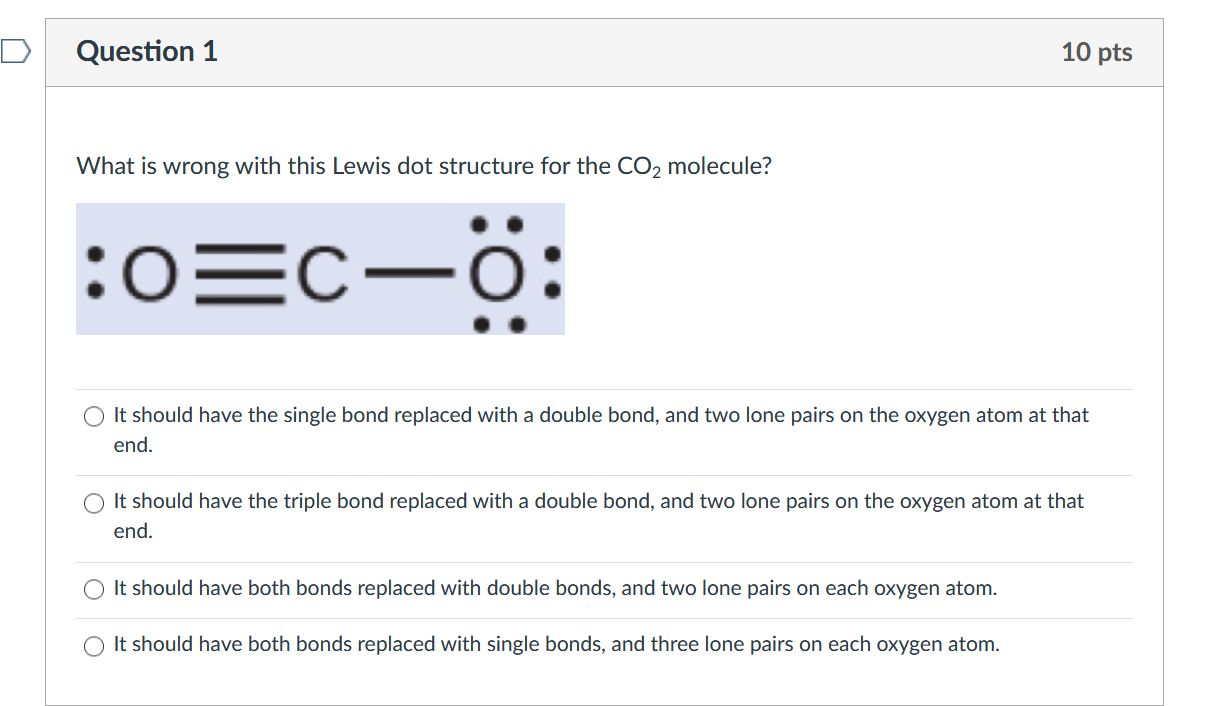
Why are the outermost electrons the only ones included in the electron dot diagram? View Answer. What is wrong with this Lewis dot structure for the CO2 molecule? (a) It should have the single ...
Which is a correct Lewis structure for carbon dioxide co2? Carbon dioxide (CO2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO2 is linear.
This structure helps in knowing the arrangement of electrons in the molecules and the shape of the molecule. To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule.
Lewis dot structure of OF2 plays an important role to determine the geometry of it because it helps to find how many bond pair and lone pair electrons OF2 molecule contains. Follow three steps to find OF2 molecular shape and its electron geometry 1. Find the Number of lone pairs present on the central atom of the OF2 lewis structure
To sketch the CO2 Lewis structure by following these instructions: Step-1: CO2 Lewis dot Structure by counting valence electrons on the carbon atom. Step-2: Lewis Structure of CO2 for counting valence electrons around the terminal oxygen atoms. Step-3: Lewis dot Structure for CO2 generated from step-1 and step-2.

What Would Be The Electron Dot Structure Of Carbon Dioxide Which Has The Formula Co2 Carbon And Its Compounds Science Class 10












.png)
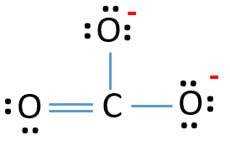








Comments
Post a Comment